Abstract
The selective carbon dioxide (CO2) absorption properties of ionic liquids (ILs) are highly pertinent to the development of methods to capture CO2. Although it has been reported that fluorinated components give ILs enhanced CO2 solubilities, it has been challenging to gain a deep understanding of the interactions occurring between ILs and CO2. In this investigation, we have utilized the soft crystalline material [Cu(NTf2)2(bpp)2] (NTf2‒ = bis(trifluoromethylsulfonyl)imide, bpp = 1,3-bis-(4-pyridyl)propane) as a surrogate for single-crystal X-ray diffraction analysis to visualize interactions occurring between CO2 and NTf2‒, the fluorinated IL component that is responsible for high CO2 solubility. Analysis of the structure of a CO2-loaded crystal reveals that CO2 interacts with both fluorine and oxygen atoms of NTf2‒ anions in a trans rather than cis conformation about the S–N bond. Theoretical analysis of the structure of the CO2-loaded crystal indicates that dispersion and electrostatic interactions exist between CO2 and the framework. The overall results provide important insight into understanding and improving the CO2 absorption properties of ILs.
Similar content being viewed by others
Introduction
Ionic liquids (ILs) have received increasing attention in the past several decades owing to their wide variety of potential industrial applications1,2,3. This is especially true for ILs that have selective carbon dioxide (CO2) absorption properties as a result of the tremendous interest in and urgency for stemming global warming by removal of green house gases from the atmosphere4. It is known that anion components of ILs significantly influence CO2 absorption capacities and that fluorine containing anions are superior CO2 absorbers as compared to those that lack this halogen5. This phenomenon is exemplified by the bis(trifluoromethylsulfonyl)imide (NTf2‒) anion, which is one of the most interesting building blocks for the construction of ILs with high CO2 absorption propensities6. Although several theories have been advanced to explain the physical interactions that take place between NTf2‒-containing ILs and CO2, some of which have been tested experimentally and by using simulations7,8,9,10,11,12, a full understanding of the interactions has not yet been gained. In particular, based on the current state of knowledge, it is still not possible to ascertain whether oxygen or fluorine is the key CO2 absorption site in NTf2‒, and to determine the nature of primary interactions occurring between this anion and this gas. The main hurdle to obtaining this information is associated with difficulties with determining the structures of ILs owing to their non-crystalline nature. In addition, the conformationally flexible structure of the NTf2‒ anion, originating from reasonably rapid rotation (barrier of 25.1 kJ mol−1) of the CF3SO2 substituent around the S–N bond (Supplementary Fig. 13), complicates elucidation of the IL-CO2 interactions.
Recently, a new class of materials, referred to as soft crystals, has attracted great attention. These materials can exist in both a highly crystalline and soft states, which can be reversibly interconverted in response to external chemical and physical stimuli13,14. Interestingly, some of the soft materials retain their single crystalline nature upon being treated with external stimuli. We envisaged that it might be possible to design and fabricate soft crystals, which contain components that are the same as or similar to those present in ILs, and that might possess the ability to absorb CO2 in synchrony with stimuli promoted structural changes. In this way, it would be possible to utilize these substances as surrogates to determine the structures of and elucidate important interactions in ILs containing absorbed CO2 using standard single-crystal X-ray diffraction techniques.
To explore this proposal, we prepared a new soft crystal [Cu(NTf2)2(bpp)2] (1), which is composed of an NTf2‒ anion and the flexible 1,3-bis(4-pyridyl)propane (bpp) ligand and Cu2+ (Fig. 1) that possess an alkyl chain and positively charged Cu2+ coordinated nitrogen centers that are typical of those present in a ILs (e.g., the alkylimidazolium and NTf2‒ containing IL in Fig. 1). In the effort described below, we demonstrated that the soft crystal 1 displays reversible absorption of CO2 associated with a stimulus-induced structural transformation, while retaining its permanent single-crystalline nature. Moreover, by using standard single-crystal X-ray diffraction techniques, we elucidated the nature of interactions with the NTf2‒ anion that are responsible for CO2 absorption.
Structures of the components of soft crystal 1 (left) and a typical IL [1-n-butyl-3-methylimidazolium][NTf2‒] containing NTf2‒ (right)6.
Results
Crystal structure of as-synthesized soft crystal
A single-crystal of 1 was prepared by using the diffusion method and a three-phase system, including a 0.1 M aqueous Cu(NTf2)2 bottom layer, a 1:1 water and MeOH, middle layer, and 0.2 M bpp in MeOH top layer. Upon standing, this system generated transparent and rod-like purple single crystals, which were shown by using the elemental analysis to have the desired atomic composition and purity. The soft crystal 1 crystallized in the monoclinic space group P2/n, containing crystallographically independent one copper ion, two NTf2‒ anions and two bpp ligands. The copper ion, surrounded by two NTf2‒ anions and four bpp ligands, exists in a distorted octahedral environment (Fig. 2a). The distance between the copper ion and NTf2‒ anions (Cu1-O1 = 2.801(2) Å) is much longer than that between the copper ion and the bpp ligands (Cu1–N1 = 2.002(2) Å, Cu1–N2 = 2.013(3) Å) as a consequence of Jahn-Teller distortion and the weak coordination ability of NTf2‒. The NTf2‒ anions exist in a trans conformation (Fig. 2b and Supplementary Fig. 1), which is known to be thermodynamically more stable than the cis conformation15. The bpp ligands bridge the copper ion to form one-dimensional (1D) chains oriented along the b-axis with a toroidal space of 6.4 Å × 12.2 Å (Fig. 2c and Supplementary Fig. 2). The 1D chains aggregate through weak CH/π interactions to form quasi two-dimensional (2D) layers with an interchain distance of 9.9 Å (Supplementary Fig. 3). In addition, NTf2‒ anions are sandwiched between the 2D layers through weak coordinative interactions, in which the distance between two neighboring layers is 8.0 Å (Supplementary Fig. 3). The 2D layers and NTf2‒ anions are densely packed through the above-mentioned weak coordinative interactions and weak hydrogen bonding interactions between the trifluoromethylsulfonyl groups of NTf2‒ and pyridyl groups of the bpp ligands (Supplementary Fig. 4). As a result, almost no pores exist in the crystal structure of 1, and the pore space calculated by using MERCURY software (probe radius: 1.2 Å, approximately grid spacing: 0.7 Å) is only 0.7%.
CO2 absorption properties and stability for water
Inspection of absorption/desorption isotherms of 1 displayed in Fig. 3a shows that almost no absorption of N2 and Ar occurs at 77 K proving that this soft crystal has a densely assembled structure. In contrast, 1 exhibits a steep increase and decrease in the amount of absorbed CO2 at P/P0 = 0.21 and 0.09, respectively, and 195 K, while N2 and Ar are not absorbed even at 195 K (Supplementary Fig. 9), which suggests that absorption of this gas takes place in association with a change between a closed and an open structure16. The saturated absorption amount of CO2 was found to be 2.03 mol mol−1 at P/P0 = 0.98 (P ≅ fugacity = 0.99 bar), which corresponds to 1.02 mol per mol of NTf2‒. This value is close to those of NTf2‒-containing CO2 absorbing ILs at 313 K and 40 bar (fugacity = 34 bar) (under this condition, absorption by NTf2‒-containing ILs is ca. 0.5 CO2 mole fraction, corresponding to 1 mol per one mol of NTf2‒)17. In order to elucidate the role of fluorinated moieties in governing CO2 absorption, we synthesized the nonfluorinated analog of [Cu(NMes2)2(bpp)2] (2), in which NMes2‒ is bis(methylsulfonyl)imide, which has a one-dimensional structure that is similar to that of 1 (Supplementary Fig. 6) and measured its absorption properties. As shown in Fig. 3a and Supplementary Fig. 10, CO2 is selectively absorbed to 2 but the threshold pressure in the absorption isotherm (P/P0 = 0.32) is higher than that of 1 (P/P0 = 0.21), indicating that the fluorinated component facilitates the CO2 absorption. A similar observation has been made in studies of the ILs with NTf2‒ and NMes2‒ anions5. The integer number (2 mol mol−1) of the amount of CO2 absorbed at saturation may be related to the free volume and/or specific absorption sites in the soft crystal 1.
a Absorption (closed symbols)/desorption (open symbols) isotherms of CO2 (black circle), N2 (blue square), Ar (green pentagon), and H2O (red rhombus) at 195 K (CO2), 77 K (N2 and Ar), and 298 K (H2O), respectively, for 1 and of CO2 (gray triangle) at 195 K for 2. b Calculated PXRD pattern of 1 from its single-crystal X-ray structure (black) and observed PXRD patterns of as-synthesized 1 (red), 1 soaked in H2O (purple) and 0.1 M HCl (blue) for 2 days.
The results of investigation of the variable-temperature CO2 absorption of 1 reveal that similar behavior is followed but that different threshold pressures exist in the absorption and desorption isotherms (Supplementary Fig. 11). Because the threshold positions are related to equilibrium pressures for the CO2 absorption reaction, their temperature dependence enables an evaluation of the enthalpy of CO2 absorption (Qst) by using the Clausius-Clapeyron equation. A plot of the natural logarithm of the threshold positions vs 1/T was found to be linear (Supplementary Fig. 12), and analysis of the slope yields a Qst value of ‒29.1 kJ mol−1. This enthalpy value is only moderate when compared with those of other CO2 absorption/adsorption materials, including a 30 wt% monoethanolamine solution (‒72 to ‒79 kJ mol−1)18, amine-functionalized porous organic polymer (‒61 kJ/mol)19 and zeolites (‒49.1, ‒50.0, and −38.0 kJ mol−1 for NaX, Na-ZSM-5 and H-ZSM-5, respectively)20. [1-n-Alkyl-3-methylimidazolium][NTf2‒] (alkyl = ethyl, butyl, and hexyl) ILs have smaller Qst values ranging from ‒11 to ‒14 kJ mol−1 because of more limited physisorption between dynamic liquid and a gas phase21. The only moderate Qst value among those of other solid adsorbents suggests that the interaction between NTf2‒ in 1 and CO2 and/or that between bpp and CO2 is not strong.
The stability and absorbability of 1 for water, which are important issues when contemplating practical uses, were also investigated. NTf2‒ anion is hydrophobic as reflected by the fact that the IL [1-n-butyl-3-methylimidazolium][NTf2‒] (Fig. 1) has a low absorbability for water and a CO2 absorption capacity that is not affected by water absorption17. The soft crystal 1 also has negligible H2O absorbability (Fig. 3a). Furthermore, soaking in neutral and acidic (0.1 M HCl) H2O for 2 d does not affect the powder X-ray diffraction (PXRD) patterns of 1 (Fig. 3b). These results show that 1 has outstanding stability and low water absorbability.
Crystal structure of CO2-loaded soft crystal
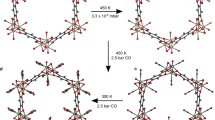
Because soft crystal 1 retains its single crystalline nature after absorption of CO2, we were able to determine the structure of the CO2-loaded crystal 1·2CO2 by using standard single-crystal X-ray diffraction analysis. The space group of 1·2CO2 was found to be C2/c, which can be considered as being a transform to a C-centered lattice with space group P2/n. The coordination environments of the copper ion in 1 and 1·2CO2 are similar (Figs. 2a and 2d), while the distance between the copper center and NTf2‒ anion is 7% shorter (Cu1′-O1′ = 2.600(2) Å) and that between the copper center and bpp ligands is slightly longer (Cu1′–N1′ = 2.015(3) Å, Cu1′–N2′ = 2.018(3) Å) in 1·2CO2 compared to 1. The toroidal shape of 1D chains in 1·2CO2 are almost unchanged (6.3 Å × 12.3 Å, Fig. 2f and Supplementary Fig. 2) in comparison with 1, and the dihedral angles of neighboring pyridine rings coordinated to the same copper ions decrease from 69°/68° in 1 to 59°/64° in 1·2CO2. Moreover, 1·2CO2 contains slightly different quasi 2D layers in comparison to 1. Specifically, each 1D chain in 1·2CO2 is inclined against the plane composed of copper ions, resulting in a slightly larger interchain distance (10.3 Å) (Supplementary Fig. 3). The interlayer space is occupied by both NTf2‒ anions and CO2 molecules, and the presence of absorbed CO2 causes a significant expansion of the interlayer distance from 8.0 to 8.6 Å (Supplementary Fig. 3). Consequently, the cell volume of 1·2CO2 is 8.5 % larger than that of 1 largely because of a 7.5% expansion of the interlayer.
As mentioned above, all NTf2‒ anions in 1 are arranged in a parallel manner in the trans conformation. However, after CO2 absorption, six NTf2‒ anions exist in a cis conformation (Supplementary Fig. 1) and are positioned at corners of hexagonal vertexes (Supplementary Fig. 5) to form a cavity which encloses two CO2 molecules (Fig. 2e). The significant changes taking place in the interlayer distance and conformation of NTf2‒ anions, along with other slight structural changes suggest that the soft crystal 1 undergoes an induced-fit type change to accommodate CO2.
Interactions that exists between the 1D chains and CO2 in 1·2CO2 are shown in Fig. 4. CO2 molecules in 1·2CO2 are disordered over two sites with occupancies of 0.66 (molecule A) and 0.34 (molecule B). The shortest contact exists between a fluorine of one NTf2‒ anion and the carbon of CO2 with F•••C distances of 2.95(2) (molecule A) and 2.68(5) Å (molecule B), which are shorter than the sums of their van der Waals radii (3.05–3.23 Å)22. On the other hand, the distances between oxygen of another NTf2‒ anion and the carbon of CO2 in molecule A (O•••C = 3.02(2) Å) and molecule B (O•••C = 3.28(5) Å) are comparable with the sums of their van der Waals radii (3.00–3.35 Å)22. Considering that molecule A has a larger occupancy, it is expected that both fluorine and oxygen in the NTf2‒ anion should play prominent roles in governing CO2 absorption. Weak hydrogen bonding interactions also exist between the bpp ligand and CO2 (H•••O and C•••O = 2.52(2) and 3.30(2) Å & 2.91(4) and 3.42(4) Å in molecule A and 2.58(4) and 3.34(4) Å & 2.83(6) and 3.34(6) Å in molecule B, Fig. 4).
The degree of quadrupole-quadrupole interactions between CO2 molecules is related to the distances between the carbons of two neighboring CO2 molecules. In 1·2CO2, the respective distances between CO2 carbons in molecules A and A, molecules A and B, and molecules B and B are 3.82(3) Å, 4.03(6) Å and 4.28(8) Å. Although the crystal structure of 1·2CO2 was determined at a temperature below the sublimation point of CO2, the intermolecular C–C distances are longer than the intermolecular distance (3.58 Å) in solid CO223. Moreover, the C–C distance between CO2 molecules in molecules B and B is greater than 4.1 Å, which is the intermolecular C–C distance in gaseous CO224. The differences in the intermolecular CO2•••CO2 distances show that a quadrupole-quadrupole interaction contributes little to stabilization of absorbed CO2 in 1·2CO2 and, consequently, demonstrate that interactions with both a fluorine and oxygen atom in the NTf2‒ anion are the most influential in governing absorption of CO2 gas by 1.
Theoretical calculations
A density functional theory calculation was performed under periodic boundary conditions to gain more information about interactions occurring between NTf2‒ anions and CO2 in 1·2CO2. The binding energy (Eb) was determined by using the equation Eb = EFramework+gas ‒ (EFramework + EGas), where EFramework + gas and EFramework are the respective energies of the complete complex 1·2CO2 and only the framework of 1·2CO2, and EGas is the energy of CO2 in a sufficiently large super cell. The calculated Eb value was found to be ‒31.3 kJ mol−1, which is consistent with the experimentally determined enthalpy of CO2 absorption. Next, an analysis of the interaction of 1·2CO2 was performed, utilizing a cluster model and an energy decomposition analysis with the natural orbitals from chemical valence theory, in order to elucidate the nature and trend of the interaction that most strongly contributes to CO2 absorption. The results (Supplementary Table 4) show that large contributors to the stabilization of the structure of 1·2CO2 are a dispersion force (‒28.7 kJ mol−1) and an electrostatic interaction (‒25.0 kJ mol−1), and that orbital interaction energy (‒13.3 kJ mol−1) is a minor contributor. This result is consistent with previous findings, which suggest that calculated Lewis acid-base binding energies between CO2 and naked anions are inversely proportional to the solubility of CO2 in ILs. Thus, Lewis acid-base interactions are not important contributors to solubilities of CO2 in ILs25.
Discussion
In Fig. 5 are displayed schematic views of the structures of soft crystal 1 formed during the CO2 absorption process. Analysis of the structures before and after CO2 absorption shows that the CO2 absorption process by 1 has features that are similar to those of ILs. Firstly, 1 preferably absorbed CO2 over other gases such as N2 and Ar. Secondly, during absorption, the lattice of 1 expands to create an interstitial space for CO226, while the distance between the cation (in this case, copper) and anion (in this case, NTf2‒) changes only slightly27. In addition, our studies have uncovered a unique feature not observed previously in studies of CO2 absorption by ILs. Specifically, we obtained direct evidence showing that the NTf2‒ anion undergoes a conformational change from the trans to cis upon CO2 binding. In ILs, the NTf2‒ anion can exist in two possible conformations (trans and cis) at room temperature with the trans form being thermodynamically more stable15,28. The reason why the conformation of the NTf2‒ anion changes when 1 absorbs CO2 is that cohesion energy is enhanced owing to the larger dipole moment of the cis form (dipole moments for trans and cis forms are 0.2–0.4 and 4.4–5.4, respectively)29. This enables the absorbed CO2 molecule interact more strongly and cooperatively with the fluorine and oxygen atoms of the two neighboring cis- NTf2‒ anions by taking full advantage of delocalized negative charges (Supplementary Table 3), in the 1D alternate arrangement of •••NTf2‒•••CO2•••NTf2‒•••CO2•••.
Schematic views of structural changes occurring in the CO2 absorption process by soft crystal 1. The upper figures show packing structures viewed along the b-axis and bottom figures show arrangements of NTf2‒ anions in the presence and absence of CO2. The 1D [Cu(bpp)2] chains are oriented along the b-axis. The dotted lines indicate intermolecular interactions between the NTf2‒ anion and CO2. The yellow meandering line shows the 1D alternate arrangement of •••NTf2‒•••CO2•••NTf2‒•••CO2•••.
Gas absorption taking place in association with a change between a closed structure and an open structure of an absorbent is dominated by a balance between the energy barrier for the structural transition of the absorbent and the enthalpy change associated with the open form absorbing the gas30,31. The fluorinated moieties contribute to decreasing the energy barrier for the structural transition because they have an ability to delocalize charge (Supplementary Table 3) and low electronic polarizability that leads to weakening intermolecular interactions32. The effect of the fluorinated groups on the gas absorption enthalpy change is likely not related to Lewis basicity because the result of this study reveal that Lewis acid-base interactions are not important contributors to solubilities of CO2. Indeed, the most basic sites in anions are close to the most acidic sites in cations, which prevents the occurrence of Lewis acid-base interactions between CO2 and the most basic sites. Although the issue requires more detailed studies, it appears that charge delocalization and low electric polarizability promoted by fluorination have respective impacts on electrostatic interactions and dispersion forces that influence CO2 absorption.
Prior to the study described above, numerous experiments have been conducted to elucidate the CO2 absorption states in ILs7,8,9,10,11,12. In this effort, using the soft crystal 1 as a surrogate, we were able to visualize interactions that occur between the NTf2‒ anion component of ILs and CO2. The pyridine rings in bpp ligands and NTf2‒ anions in 1 synergistically constrain CO2 molecules in the crystal. The NTf2‒ anion contains the primary absorption sites for CO2 comprised of a trifluoromethyl fluorine and sulfonyl oxygen atom. The bpp ligand supports the NTf2‒-CO2 interaction through the formation of a weak phenyl hydrogen-CO2 interaction. As a result, a dispersion force and electrostatic interaction are the main contributors to stabilization of the CO2-absorbed complex. Furthermore, a conformational change of the NTf2‒ anions from trans to cis occurring upon CO2 capture by 1 also contributes to the absorption capacity. Of course, we understand that the observations made in this investigation have been made using a surrogate and not under real IL conditions especially because of differences in temperature and fluidity of the system. In addition, ILs with the NTf2‒ anion are not practical for CO2 separation because the perfluorinated groups containing NTf2‒ anion is not an environment-friendly. Nevertheless, the findings should not only increase the level of understanding of CO2 absorption by ILs but also provide new approaches to probe interactions between ILs and CO2 and to design new systems for CO2 separation applications.
Methods
Synthesis
Commercially available reagents were used as received without further purification. Cu(NTf2)2 and Cu(NMes2)2 were synthesized using previously described procedures28,33.
A single-crystal of [Cu(NTf2)2(bpp)2] (1) was prepared by using a diffusion method in the straight glass tube containing a bottom layer of 0.1 M aqueous Cu(NTf2)2, a middle layer of 1:1 water and MeOH, and a top layer of 0.2 M bpp in MeOH. After 1 week, the formed transparent and rod like purple single crystals were separated by filtration, washed with water, and dried in the atmosphere. Elemental analysis was used to confirm the atomic composition and purity of the as-synthesized single crystals. Elemental analysis (%) calcd for C30H28Cu1F12N6O8S4: C 35.31, H 2.77, N 8.24, F 22.34, S 12.57. Found: C 34.86, H 2.57, N 8.04, F 22.77, S 12.69. The thermogravimetric curve and Fourier transform infrared spectrum of 1 are shown in Supplementary Figs. 7 and 8.
A single-crystal of {[Cu(NMes2)2(bpp)2]·3H2O} (2·3H2O) was prepared by using a solvent evaporation method in the beaker containing 0.1 M aqueous Cu(NMes2)2 and 0.2 M bpp in MeOH. After 1 week, the formed transparent and plate like blue single crystals were separated by filtration, washed with MeOH, and dried in the atmosphere. This crystal lost a part of hydrated water during drying, which was confirmed using elemental analysis. Elemental analysis (%) calcd for C30H44.4Cu1N6O10.2S4 (2·2.2H2O): C 42.69, H 5.30, N 9.96. Found: C 42.20, H 4.98, N 9.76. A single-crystal of [Cu(NMes2)2(bpp)2] (2) was prepared by drying 2·3H2O under N2 flow at 373 K for 30 min.
Single-crystal X-ray diffraction analysis of 1, 1·2CO2, and 2
Crystal data for 1 were collected at 173 K on a RIGAKU RAXIS-RAPID imaging-plate diffractometer with a graphite-monochromated Mo-Kα radiation (λ = 0.71075 Å). Crystal data for 2 were collected at 293 K on a RIGAKU XtaLab Synergy-R with a multi-mirror monochromated Mo-Kα radiation (λ = 0.71075 Å). The structures were solved by using direct method (SHELXT)34 and refined by using full-matrix least-squares techniques on F2 using SHELXL-201835. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were located at geometrically calculated positions and refined using a riding model. Olex2 was used as a graphical user interface36. Crystallographic data are shown in Supplementary Tables 1 and 2 and Supplementary Data 1 and 3.
The structural determination of 1·2CO2 was carried out using the following procedure. A single crystal of 1 was placed at the bottom of a glass capillary, which was then adhered to the base using glue. The base was installed on the absorption equipment (BELSORP-mini, MicrotracBEL Corp.) and the crystal was heated at 373 K for 30 min under a vacuum as the pre-treatment. After pre-treatment, CO2 gas at 40 kPa was added and the capillary was cut and sealed quickly. The capillary containing single crystal sample and CO2 gas was placed in the single-crystal X-ray diffractometer with a cryogenic unit, and cooled at a rate of 1 K min−1 from 293 K to 173 K, and then held at 173 K for 4 h. X-ray diffraction measurements were then made at 173 K on a RIGAKU XtaLAB P200 with a multi-mirror monochromated Cu-Kα radiation (λ = 1.5418 Å). Data analysis was performed in the manner described for 1. Crystallographic data are shown in Supplementary Table 1 and Supplementary Data 2.
Gas absorption isotherm experiments
CO2 (195 K), N2 (77 K and 195 K) and Ar (77 K and 195 K) absorption/desorption isotherms were measured by using a BELSORP-max (MicrotracBEL Corp.). Water absorption/desorption isotherms were measured at 298 K by using a BELSORP-aqua (MicrotracBEL Corp.). Before the measurement, the sample was heated at 373 K in a vacuum overnight for activation by BELPREP-vac (MicrotracBEL Corp.). High-pressure CO2 absorption/desorption isotherms were measured by using a BELSORP-HP (MicrotracBEL Corp.). Activation of the sample was performed on a BELSORP-HP at 373 K in a vacuum.
Theoretical calculations
For determining the S–N bond rotational barrier in NTf2‒, relax scan calculations were performed using gaussian 09 Rev. E.01 software at the B3LYP/aug-cc-pVDZ level of theory37. A PBE functional38, with projector augmented wave potentials and van der Waals interaction corrected by using a D3 scheme39 to optimize the atomic positions of 1, CO2 and 1·2CO2, was used to obtain an estimate of the binding energy of CO2 (Supplementary Fig. 14). In all cases, spin polarized calculations were employed under periodic boundary condition and Γ-point approximation using the Vienna Ab initio Simulation Package (VASP)40,41,42,43 with the cut-off energy of 400 eV. The atomic charges were evaluated based on Bader analysis (Supplementary Table 3)44,45,46,47. In a manner that is similar to other reported approaches48,49,50, after optimization of 1·2CO2, a model consisting of one CO2, three NTf2− and three (4-pyridyl)butane, which are protonated instead of bonded to Cu2+ (Supplementary Fig. 15), was employed to investigate the nature of the interaction between CO2 and 1. The positions of added H atoms were optimized with a PBE functional using Amsterdam Density Functional 2018 program51. A standard triple-ζ STO basis set with two sets of polarization functions (TZ2P)52 and a Grimme D3 type dispersion correction were applied to all atoms in the model. The model structure was optimized and analyzed by using the energy decomposition method53 combined with the natural orbitals for chemical valence theory54,55 with the same level of theory as that used for optimization of H atoms.
Data availability
Crystallographic data for the structures reported in this manuscript have been deposited at the Cambridge crystallographic Data Centre under deposition numbers CCDC 1981481 (1, Supplementary Data 1), 1981483 (1·2CO2, Supplementary Data 2), and 2021731 (2, Supplementary Data 3). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other relevant data that support the findings of this study are available within the manuscript and its Supplementary Information, or from the corresponding author upon reasonable request.
References
Seddon, K. R. Ionic liquids for clean technology. J. Chem. Technol. Biotechnol. 68, 351–356 (1997).
Zhao, D. et al. Ionic liquids: applications in catalysis. Catal. Today 74, 157–189 (2002).
Plechkova, N. V. & Seddon, K. R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 37, 123–150 (2008).
Lei, Z. et al. Gas solubility in ionic liquids. Chem. Rev. 114, 1289–1326 (2014).
Pringle, J. M. et al. The effect of anion fluorination in ionic liquids-physical properties of a range of bis(methanesulfonyl)amide salts. N. J. Chem. 27, 1504–1510 (2003).
Anthony, J. L. et al. Anion effects on gas solubility in ionic liquids. J. Phys. Chem. B 109, 6366–6374 (2005).
Kelley, S. P. et al. Understanding carbon dioxide solubility in ionic liquids by exploring the link with liquid clathrate formation. Chem. Eur. J. 23, 14332–14337 (2017).
Rao, S. S. & Gejji, S. P. CO2 Absorption using fluorine functionalized ionic liquids: interplay of hydrogen and σ-hole interactions. J. Phys. Chem. A 120, 1243–1260 (2016).
Damas, G. B. et al. A quantum chemistry study for ionic liquids applied to gas capture and separation. J. Phys. Chem. B 118, 9046–9064 (2014).
Ramdin, M. et al. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 51, 8149–8177 (2012).
Hu, Y. F. et al. The molecular characteristics dominating the solubility of gases in ionic liquids. Chem. Soc. Rev. 40, 3802–3823 (2011).
Lourenço, T. C. et al. Insights on the solubility of CO2 in 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide from the microscopic point of view. Environ. Sci. Technol. 47, 7421–7429 (2013).
Horike, S. et al. Soft porous crystals. Nat. Chem. 1, 695–704 (2009).
Kato, M. et al. Soft crystals: flexible response systems with high structural order. Chem. Eur. J. 25, 5105–5112 (2019).
Lassègues, J. C. et al. Raman and ab initio study of the conformational isomerism in the 1-ethyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide ionic liquid. J. Raman Spectrosc. 38, 551–558 (2007).
Kitaura, R. et al. Porous coordination-polymer crystals with gated channels specific for supercritical gases. Angew. Chem., Int. Ed. 42, 428–431 (2003).
Aki, S. N. V. K. et al. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J. Phys. Chem. B 108, 20355–20365 (2004).
Song, H.-J. et al. Simplified estimation of regeneration energy of 30 wt % sodium glycinate solution for carbon dioxide absorption. Ind. Eng. Chem. Res. 47, 9925–9930 (2008).
Lu, W. et al. Cost-effective synthesis of amine-tethered porous materials for carbon capture. ChemSusChem 8, 410–410 (2014).
Dunne, J. A. et al. Calorimetric heats of adsorption and adsorption isotherms. 1. O2, N2, Ar, CO2, CH4, C2H6, and SF6 on silicalite. Langmuir 12, 5888–5895 (1996).
Dębski, B. et al. Thermodynamic interpretation and prediction of CO2 solubility in imidazolium ionic liquids based on regular solution theory. J. Mol. Liq. 291, 110477 (2019).
Batsanov, S. S. Van der Waals radii of elements. Inorg. Mater. 37, 871–885 (2001).
Brigot, N. et al. The structure of the carbon dioxide dimer. Chem. Phys. Lett. 49, 157–159 (1977).
Mannik, L. et al. An Infrared spectrum of CO2 dimers in the “Locked” configuration. Can. J. Phys. 49, 3056–3057 (1971).
Bhargava, B. & Balasubramanian, S. Probing anion–carbon dioxide interactions in room temperature ionic liquids: gas phase cluster calculations. Chem. Phys. Lett. 44, 242–246 (2007).
Babarao, R. et al. Understanding the high solubility of CO2 in an Ionic Liquid with the Tetracyanoborate anion. J. Phys. Chem. B 115(32), 9789–9794 (2011).
Lourenço, T. C. et al. Local environment structure and dynamics of CO2 in the 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide and related ionic liquids. J. Chem. Phys. 146, 104502 (2017).
Fujii, K. et al. Conformational equilibrium of bis(trifluoromethanesulfonyl) imide anion of a room-temperature ionic liquid: Raman spectroscopic study and DFT calculations. J. Phys. Chem. B 110, 8179–8183 (2006).
Hoogerstraete, T. V. et al. Crystal structures of low-melting ionic transition-metal complexes with N-alkylimidazole ligands. CrystEngComm 14, 4902–4911 (2012).
Numaguchi, R. et al. Molecular simulation of capillary phase transitions in flexible porous materials. J. Chem. Phys. 138, 054708 (2013).
Gurkan, B. et al. Molecular design of high capacity, low viscosity, chemically tunable ionic liquids for CO2 capture. J. Phys. Chem. Lett. 1, 3494–3499 (2010).
Noro, S. et al. Controlling the gate-sorption properties of solid solutions of Werner complexes by varying component ratios. Dalton Trans. 49, 9438–9443 (2020).
Henschel, D. et al. Metall(II)-dimethylamid-tetrahydrate: das dimethylamin-anion als einzähniger O-ligand und tetrafunktioneller Wasserstoffbrucken-Akzeptor in den isotypen Komplexen [M(H2O)4{(CH3SO2)2N}2] mit M = magnesium, nickel, kupferund zink. Z. Anorg. Allg. Chem. 622, 1065–1075 (1996).
Sheldrick, G. M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. A71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Cryst. C71, 3–8 (2015).
Dolomanov, O. V. et al. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Frisch, M. J. et al. Gaussian 09, Revision E.01 (Gaussian, Inc., Wallingford CT, 2009).
Perdew, J. P. et al. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S. et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. J. Comput. Mat. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Tang, W. et al. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter. 21, 084204 (2009).
Sanville, E. Improved grid-based algorithm for Bader charge allocation. J. Comp. Chem. 28, 899–908 (2007).
Henkelman, G. A. et al. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Yu, M. & Trinkle, D. R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 134, 064111 (2011).
Sillar, K. et al. Ab Initio Study of Hydrogen Adsorption in MOF-5. J. Am. Chem. Soc. 131, 4143–4150 (2009).
Valenzano, L. et al. Heats of adsorption of CO and CO2 in metal–organic frameworks: quantum mechanical study of CPO-27-M (M = Mg, Ni, Zn). J. Phys. Chem. C. 115, 21777–21784 (2011).
Simpson, S. et al. Modulating bond lengths via backdonation: a first-principles investigation of a quinonoid zwitterion adsorbed to coinage metal surfaces. J. Phys. Chem. C. 120, 6633–6641 (2016).
Velde, G. T. et al. Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001).
Lenthe, E. V. et al. Relativistic regular two‐component Hamiltonians. Int J. Quantum Chem. 57, 281–293 (1996).
Ziegler, T. & Rauk, A. On the calculation of bonding energies by the Hartree Fock Slater method. Theor. Chim. Acta 46, 1–10 (1977).
Michalak, A. et al. Bond orbitals from chemical valence theory. J. Phys. Chem. A 112, 1933–1939 (2008).
Mitoraj, M. & Michalak, A. Natural orbitals for chemical valence as descriptors of chemical bonding in transition metal complexes. J. Mol. Model. 13, 347–355 (2007).
Acknowledgements
This study was supported financially by a Grant-in-Aid for Scientific Research (No. 18K19864) from MEXT, Japan. One of the authors (X.Z.) was supported by The Ministry of Education, Culture, Sports, Science, and Technology through Program for Leading Graduate Schools (Hokkaido University “Ambitious Leader’s Program”).
Author information
Authors and Affiliations
Contributions
X.Z. and K.F. synthesized and characterized the soft crystals, and measured the absorption isotherms. X.Z. carried out the single-crystal X-ray diffraction analysis for CO2-loaded crystal. H.S. supported the structural analysis of the CO2-loaded crystal. K.T. supported the single-crystal X-ray diffraction analysis. Y.H. and J.P. performed the theoretical calculations. All authors contributed to the writing and editing of the manuscript. S.N. and T.N. conceived the project and directed the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, X., Fukuhara, K., Hijikata, Y. et al. Understanding the interactions between the bis(trifluoromethylsulfonyl)imide anion and absorbed CO2 using X-ray diffraction analysis of a soft crystal surrogate. Commun Chem 3, 143 (2020). https://doi.org/10.1038/s42004-020-00390-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-020-00390-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.