Abstract
β-amino acid esters are important scaffolds in medicinal chemistry and valuable building blocks for materials synthesis. Surprisingly, the waste-free construction of such moieties from readily available or renewable starting materials has not yet been addressed. Here we report on a robust and versatile method for obtaining β-amino acid esters by direct amination of β-hydroxyl acid esters via the borrowing hydrogen methodology using a cooperative catalytic system that comprises a homogeneous ruthenium catalyst and an appropriate Brønsted acid additive. This method allows for the direct amination of esters of 3-hydroxypropionic acid, a top value-added bio-based platform chemical, opening a simple route to access β-amino acid esters from a range of renewable polyols including sugars and glycerol.
Similar content being viewed by others
Introduction
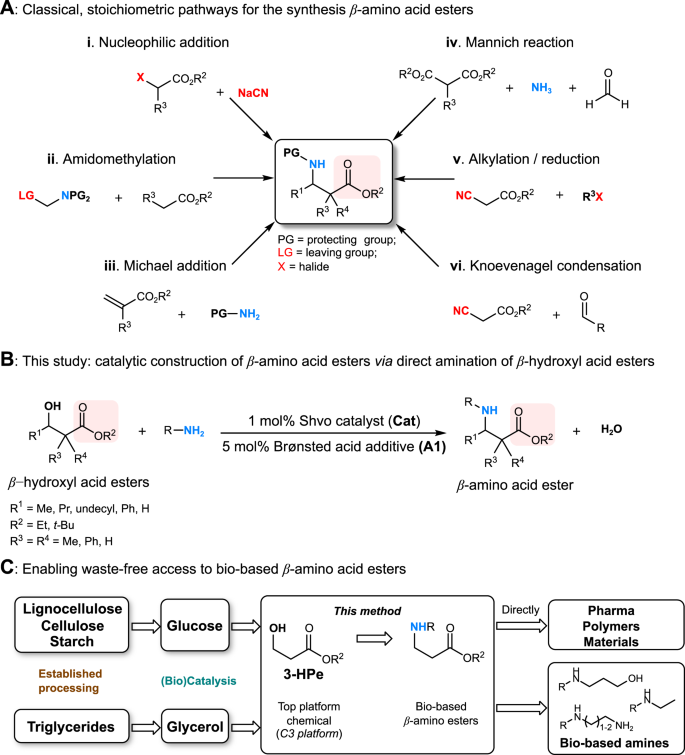
β-Amino acid esters are privileged structural motifs in a wide variety of biologically active compounds1 and indispensable building blocks for the synthesis of β-peptides2,3 and β-lactam antibiotics4,5. While β-amino acid moieties can be readily constructed by classical stoichiometric methods, these approaches frequently involve the use of toxic reagents and generate significant amounts of waste (Fig. 1a)6,7,8,9. Surprisingly, no waste-free catalytic methods, capable of creating β-amino acid scaffolds via direct coupling of β-hydroxyl acids or esters with amines, have been reported to date. Nonetheless, for targeting pharmaceutical compounds as well as functional materials and polymers, a clean synthetic approach would be certainly preferred (Fig. 1b)10. Moreover, such atom-economic method would enable the unprecedented, direct catalytic amination of important bio-based β-hydroxyl acid ester building blocks.
Strategies to access β-amino acid esters. a Classical, stoichiometric pathways; b novel catalytic method for N-alkylation of β-amino acid esters via the hydrogen borrowing strategy established here; c new route to bio-based β-amino acid esters from renewable polyols and subsequent transformation to valuable bio-based building blocks
3-Hydroxypropionic acid (3-HP) has been identified as one of the top twelve value-added renewable platform chemicals11,12,13, hence there is a clear demand for its diversification beyond already existing targets11,12,13,14. Several chemo- and biocatalytic routes have been proposed for the conversion of 3-HP and its esters to chemical intermediates11,12,13,14, including acrylonitrile15. Interestingly, among these (de)functionalization pathways, the direct and selective amination of the (3-HP) alcohol moiety has not been recognized or achieved yet. In recent years, much attention has been devoted to the development of industrially relevant, scalable methods for the production of 3-HP and its ethyl ester from renewable polyols (Fig. 1c)12,15,16,17 Thus realizing the above mentioned one-step catalytic amination would create access to valuable synthetic β-amino acid esters from diverse renewable sugar feedstocks, including non-edible lignocellulosic agricultural or forestry waste materials18, as well as glycerol, the major byproduct of biodiesel production19.
An attractive method for carrying out the desired catalytic C–N bond formation is the direct amination of alcohols via the borrowing hydrogen approach (Fig. 2a)20,21,22,23. Despite tremendous progress24,25,26,27, methodology development in the field has generally overlooked the use of potentially strongly coordinating substrates and no examples on β-hydroxyl acids or derivatives have been reported. In the recent pioneering work, Yan and co-workers have reported the first example of catalytic amination of biomass-derived α-hydroxyl acids with ammonia, using heterogeneous Ru-based catalysts28. This work pioneered sustainable pathways from sugars to α-amino acids by a tandem biocatalysis/heterogeneous catalysis approach. Earlier, Beller described the first example of catalytic amination of α-hydroxyl amides with amines using [Ru3(CO)12]/DCPE29. This study also included methyl 2-hydroxypropanoate as substrate, but only the corresponding α-amino amide was formed, indicating low ester functional group tolerance under the reported conditions.
Here we set to realize the catalytic amination of β-hydroxyl acid esters, including esters of the bio-based 3-hydroxypropionic acid.
Results
Establishment of the reaction conditions
This transformation is expected to be challenging because of side reactions such as intermolecular transesterification, partial ester hydrolysis or β-amino acid amide formation. Moreover, the β-hydroxyl acids or corresponding β-ketoacid/β-iminoacid intermediates (Fig. 2a) may form chelating complexes with the homogeneous catalyst, blocking coordination sites necessary for efficient catalysis30,31,32. Therefore, the desired transformation requires a robust catalytic system with great functional group tolerance. Very recently, we developed the first N-alkylation of unprotected α-amino acids with alcohols using the Ru-based Shvo’s catalyst33. This robust and base-free catalytic system appeared as excellent starting point for the synthesis of β-amino acid esters from β-hydroxyl acid esters and various amines (Fig. 1b). We started our investigation using ethyl 3-hydroxybutanoate and p-anisidine, with the Ru-based Shvo’s catalyst. Very poor substrate conversion was seen even at 120 °C, and the desired product was observed only in traces beside a small amount of imine (Table 1, entry 1).
In view of the possible side reactions and with the aim to keep low catalyst loading and mild reaction conditions, we explored alternative ways of enhancing reactivity. Achiral and chiral Brønsted acids have emerged as powerful tools in a wide variety of transformations34,35,36,37,38,39,40,41,42. In particular, the use of Brønsted acids in combination with transition metal catalysts have shown beneficial in hydrogenation reactions, such as Ru34, Ir35 and Fe-catalyzed hydrogenation of imines36, as well as reductive amination37. Interestingly, recently Zhao has demonstrated the enantioselective amination of alcohols by a cooperative catalytic system comprising an iridium complex and an appropriate chiral phosphoric acid, via the borrowing hydrogen methodology38. Thus, inspired by the remarkable achievements in cooperative transition-metal and Brønsted acid catalysis34,35,36,37,38,39,40,41,42 we have applied diphenyl phosphate (A1) as a Brønsted acid additive, assuming that it may facilitate imine reduction by bifunctional catalysis, and in addition potentially enhance imine formation, both steps involved in the borrowing hydrogen cycle (Fig. 2a).
Indeed, perfect (>99%) conversion and selectivity (>99%) were achieved using diphenyl phosphate (A1) and Cat at 120 °C (Table 1, entry 2). The high level of product selectivity shows that under these carefully selected conditions, the tendency for β-elimination is overcome in favor of dehydrogenation and imine formation. Further lowering the reaction temperature or catalyst amount have not proven beneficial (Table 1, entries 3–5). A blank reaction in the absence of catalyst and additive, or just with A1, gave no detectable conversion (Table 1, entries 6–7). Solvent screening showed moderate success (Table 1, entries 8–11). Decreasing the amount of alcohol to 1.5 and 1 equivalents (Table 1, entries 12–13) gradually declined conversion therefore for future study an alcohol: amine ratio of 2:1 was kept.
Additional in situ 1D and 2D 1H NMR (Supplementary Figs. 1–8) and GC-FID and GC-MS studies (Supplementary Figs. 9–10) of the amination of ethyl 3-hydroxybutanoate (1a) and ethyl 3-hydroxy-2,2-dimethylpropanoate (shown later, 1e) with p-anisidine (2a) in presence of Cat with/without diphenyl phosphate additive (A1) were performed. All key intermediates (Supplementary Figs. 1–4, 7–10), such as the corresponding imine (3’ea), enamine (3”aa) and ketone (1’a), were detected that affirmed the proposed borrowing hydrogen mechanism Fig. 2a. Deuterium incorporation experiments using the separately prepared, selectively D-labeled key substrate ethyl 3-hydroxyhexanoate-3-d (1b-d1) and applying the simpler substrate, benzyl alcohol-α,α-d227 (see Supplementary Note 2) showed deuterium transfer from the substrate to the amine product in accordance with a borrowing hydrogen mechanism. Furthermore amination of chiral alcohols (see Supplementary Note 3), namely ethyl (S)-3-hydroxybutyrate ((S)-1a) and ethyl (R)-3-hydroxybutyrate ((R)-1a) with p-anisidine (2a) lead to racemic amine products, as further evidence for the existence of the borrowing hydrogen pathway over an ionic mechanism43. The former pathway proceeds through a loss of the chirality of the substrate alcohol by its dehydrogenation to furnish the corresponding achiral carbonyl compound.
Role of the Brønsted acid additive
Gratifyingly, additional 1H NMR experiments also revealed the existence of an imine (3’aa) - enamine (3”aa) equilibrium and the shift of this equilibrium in the presence of additive A1 toward the more reactive imine 3’aa form (Fig. 3a).
a An imine-enamine equilibrium. Herein 1H NMR spectrum of the 3-(4-methoxyphenylamino)-but-2-enoic acid ethyl ester (3”aa) in presence of diphenyl phosphate additive (A1) is displayed. b Proposed adducts involved in cooperative catalysis. Details of the 31P NMR investigation are reported in Supplementary Fig. 12, Supplementary Note 1
More experiments were conducted to further elaborate on the role of the acid additive in the crucial imine formation and imine hydrogenation steps of the hydrogen borrowing cycle. Reactions between ketone (1’a) and p-anisidine (2a) with and without additive A1 were conducted, showing a beneficial effect of the additive on the imine formation step, as expected: full conversion and >99% selectivity were achieved with A1 while 64% conversion and 22% selectivity were seen without A1. Conducting this reaction step separately also shows the advantage of the full borrowing hydrogen cycle that starts from the alcohol directly and results in the stable amine product. Advantageously, in this case the ketone and apparently labile imine intermediates are kept at low concentration thereby minimizing the possibility for side reactions. Next, we examined the hydrogenation of the enamine (3”aa), which was obtained via synthetic procedure44, in the presence of 1 mol% Shvo’s catalyst (Cat) with/without acid co-catalyst (A1) (Fig. 2b). The excellent, 99% 3aa yield in the presence of A1 compared to the lower 61% 3aa yield obtained in the absence of A1 underscores its beneficial effect on the rate of imine hydrogenation.
To further understand how this rate enhancement occurs, and to gain more insight into a possible cooperative catalysis by Cat-A1, in situ 31P NMR spectroscopic investigations using toluene-d8 as solvent at 60 °C were conducted (Supplementary Fig. 12, Supplementary Note 1). These experiments have provided support for the formation of adducts between Shvo’s complex (Cat) and diphenyl phosphate (A1) (Fig. 3b, Complex 1) and between the imine 3’aa, Shvo’s complex (Cat) and diphenyl phosphate (A1) (Fig. 3b, Complex 2) desired in cooperative catalysis36. The interaction between enamine 3”aa and A1 was also confirmed (Fig. 3b, Adduct 3). We assume that in the absence of A1, tautomerization of the imine 3’aa (formed during the borrowing hydrogen cycle) to the corresponding enamine 3”aa would take place, while in the presence of Cat and A1, 3’aa is rapidly reduced to the desired β-amino acid ester (3) via the ruthenium-amine complex (Fig. 3b, Complex 2).
Scope of the methodology
Next, the scope and limitation of the newly established method were explored. A wide range of anilines were effectively coupled with ethyl 3-hydroxybutanoate (Fig. 4, Supplementary Table 1). With anilines bearing electron-donating substituents (2a-f), including those with bulky groups (2e, 2 f), 48–87% isolated product yields were achieved. Anilines with electron-withdrawing substituents (2g-l) also showed generally high reactivity affording products 3ag-al in 47–87% isolated yield. Functional groups such as –NO2, –CN, –CH3COOCH3 were well-tolerated under the reaction conditions. Notably, also when (2p) and (2r) containing heterocycles were examined, the alkylated β-amino acid esters (3ap, 3ar) were obtained in 78% and 44% isolated yield, respectively.
Scope with variation of the amine substrate. General reaction conditions: General Procedure (see Supplementary information, page 1-3), 1 mmol of 1a, 0.5 mmol of 2a-r, 1 mol% Shvo complex (Cat), 5 mol% additive (A1), 2 mL toluene, 18 h, 120 °C, under argon, full conversion unless otherwise indicated, isolated yields are presented. a 95% conversion. b 94% conversion. See also Supplementary Table 1
Furthermore, we examined different β-hydroxyl acid esters as coupling partners to p-anisidine (2a)/p-bromoaniline (2i) (Fig. 5, Supplementary Table 2). Employing β-hydroxyl acid esters with bulky aliphatic substituents at β-position (1b, 1c) delivered the desired β-amino acid esters (3ba, 3bi, 3ca and 3ci) in good yields (79%, 68%, 78%, and 54%, respectively) while with ethyl 3-hydroxy-3-phenylpropanoate (1d) generally lower isolated yields were obtained (3da-3di, 43–48%). Excellent results (81–96%) were obtained with ethyl 3-hydroxy-2,2-dimethylpropanoate (1e) comprising two methyl substituents in the α-position (3ea-3ei, 81–96%). In comparison, 1f bearing an α-phenyl substituent displayed moderate results (3fa-3fi, 33-59%).
Scope with variation of the β-hydroxyl acid ester substrate. General reaction conditions: General Procedure (see Supplementary information, page 1-3), 1 mmol of 1a-f, 0.5 mmol of 2a or 2i, 1 mol% Shvo’s complex (Cat), 5 mol% additive (A1), 2 mL toluene, 18 h, 120 °C, under argon, full conversion unless otherwise indicated, isolated yields are presented. a 48 h. b 12 mmol of 1e, 6 mmol of 2i, 1 mol% Shvo’s complex (Cat), 5 mol% additive (A1), 5 mL toluene, 18 h, 120 °C, under argon. c 79% conversion. d 83% conversion. See also Supplementary Table 2
Having a highly selective method in hand for obtaining 3ei, the power of our developed catalytic method was demonstrated in the two-step, gram-scale synthesis of a β-lactam (4ei, Fig. 6). A 12-fold upscale of the amination of 1e with p-bromoaniline 2i (Fig. 5) furnished the desired β-amino acid ester (3ei) with excellent isolated yield (86%), which was subsequently cyclized following a known literature procedure (Fig. 6)45.
Bio-based β-amino acid esters from 3-hydroxypropionates
Finally, to demonstrate the feasibility of this method for obtaining renewable β-amino acid esters in a remarkably simple manner, we turned our attention to the direct catalytic amination of esters of bio-based 3-HP, identified as one of the Top 12 value-added platform chemicals11,13. It is important to mention that the ethyl ester of 3-HP can be directly obtained from renewable resources, similarly to the acid 3-HP itself15,16. Herein we have investigated the use of commercially available tert-butyl 3-hydroxypropionate (1i) as well as ethyl 3-hydroxypropanoate (1j) as substrates. Gratifyingly, both (1i) as well as (1j) were smoothly aminated with 2a-o using the methodology developed herein (Fig. 7, Supplementary Table 3). Notably, the reaction conversion was significantly decreased in the absence of the additive A1 (Supplementary Table 3, entry 7), confirming the necessity of the catalytic system designed above. Interestingly, selective double N-alkylation of 2a with 1i-j was easily achieved by doubling the catalyst amount to 2 mol%, showing modularity of the method. The isolated yields of products obtained from the 3-HP esters, were somewhat lower compared to previously tested substrates (especially 3-hydroxy-2,2-dimethylpropanoate (1e)), thus the possibility of side reactions cannot be ruled out, although no side products (e.g. amides) were detectable by GC-MS or GC-FID methods. Hydrolysis of the 3-HP esters or the product β-amino acid esters to the corresponding carboxylic acids would be a possible pathway. Interestingly, with substrate 1e, minimal amount of side products attributable to intermolecular transesterification processes (Supplementary Figs. 9–10) were seen. Similar reactivity may also be expected starting from the bio-based 3-HP esters 1i or 1j albeit presumably toward higher molecular weight analogs due to the decreased steric hindrance of the primary alcohol moiety.
Novel route to bio-based β-amino acid esters via direct catalytic amination of 3-HP esters. General reaction conditions: General Procedure (see Supplementary information, page 1-3), 1 mmol of 1i-j, 0.5 mmol of 2a-o, 1 mol% Shvo’s complex (Cat), 5 mol% additive (A1), 2 mL toluene, 18 h, 120 °C, under argon, isolated yields are presented. a 48 h. b 1 mmol of 1i-j, 0.5 mmol of 2a, 2 mol% Shvo’s complex (Cat), 5 mol% additive (A1), 2 mL toluene, 48 h, 120 °C, under argon
Discussion
In summary, we have achieved the first direct catalytic coupling of β-hydroxyl acid esters with amines to construct β-amino acid esters by cooperative catalysis using the combination of the Shvo’s catalyst and a Brønsted acid additive. The methodology is highly atom-economic, demonstrates a broad scope, excellent functional-group tolerance and potential application for the synthesis of β-lactams. Notably, the method allows for catalytic amination of a commercially available ester of 3-hydroxypropionic acid, an important bio-based platform chemical, opening an entirely new possibility to access valuable β-amino acid scaffolds from several classes of abundant renewable resources. The obtained β-amino acid esters can be applied as value-added building blocks or further transformed to a variety of bio-based amines, diamines, amino-alcohols usable in the fine chemical, materials or polymer chemistry sectors. The novel cooperative catalytic system presented should be broadly applied, in the future, for the waste-free amination of other highly oxygenated renewable building blocks.
Methods
Synthesis and characterization
For general information about used chemicals, analytical methods, synthetic procedures, please see Supplementary Methods. 1H, 13C, 31P NMR spectra, GC-FID, GC-MS chromatograms related to the mechanism of the observed catalytic reaction are available in Supplementary Figs. 1–16 and Supplementary Notes 1–3. Full procedures for synthetic transformations to compounds 3aa-3jo, 4ei are available in Supplementary Tables 1–3. 1H, 13C NMR spectra of purified compounds are available in Supplementary Figs. 17–58.
General procedure for the preparation of β-amino acid esters
An oven-dried 20 mL Schlenk tube, equipped with a stirring bar, was charged with amine (0.5 mmol, 1 equiv.), β-hydroxyl acid ester (1 mmol, 2 equiv.), Shvo’s catalyst (0.005 mmol, 1 mol%), diphenyl phosphate (0.025 mmol, 5 mol%) and toluene (as a solvent, 2 mL). Solid materials were weighed into the Schlenk tube under air. Then the Schlenk tube was subsequently connected to an argon line and vacuum-argon exchange was performed three times. Liquid starting materials and solvent were charged under an argon stream. The Schlenk tube was capped and the mixture was rapidly stirred at room temperature for 1 min, then was placed into a pre-heated oil bath at 120 °C and stirred for a given time (typically, 18 h). Then, the reaction mixture was cooled down to room temperature. After taking a sample (app. 0.5 mL) for GC analysis, the crude mixture was filtered through silica gel, eluted with ethyl-acetate, and concentrated in vacuo. The residue was purified by flash column chromatography to provide the pure β-amino acid ester.
General procedure for in situ 31P NMR study
An oven-dried 20 mL Schlenk tube, equipped with a stirring bar, was charged (depending on the experiment) with Shvo’s catalyst (0.02 mmol, 1 equiv.), diphenyl phosphate (0.02 mmol, 1 equiv.) and/or 3-(4-methoxyphenylamino)-but-2-enoic acid ethyl ester (3”aa, 0.02 mmol, 1 equiv.) and toluene-d8 (as a solvent, 1 mL). Solid materials were weighed into the Schlenk tube under air. Then the Schlenk tube was subsequently connected to an argon line and vacuum-argon exchange was performed three times. Liquid starting materials and solvent were charged under an argon stream. The Schlenk tube was capped and the mixture was rapidly stirred at room temperature for 1 min, then was placed into a pre-heated oil bath (60°C) and stirred for 15 min. Then, the reaction mixture was cooled down to room temperature. Preparing the sample, 0.6 mL of the reaction mixture was placed to a J-Young NMR tube under argon. All spectra were recorded using Bruker Avance NEO 600 machine.
General procedure for the hydrogenation of N-aryl enamine
An oven-dried 20 mL Schlenk tube, equipped with a stirring bar, was charged with Shvo’s catalyst (0.002 mmol, 1 equiv.), diphenyl phosphate (0.01 mmol, 1 equiv.) or 3-(4-methoxyphenylamino)-but-2-enoic acid ethyl ester (3”aa, 0.2 mmol, 1 equiv.) and toluene (as a solvent, 2 mL). Solid materials were weighed into the Schlenk tube under air. Then the Schlenk tube was subsequently connected to an argon line and vacuum-argon exchange was performed three times. Liquid starting materials and solvent were charged under an argon stream. The Schlenk tube was capped and the mixture was rapidly stirred at room temperature for 1 min. At the same time the pre-dried autoclave, equipped with the stirring bar, was purged three times with hydrogen. Under the stream of hydrogen, the reaction mixture was transferred from the Schlenk tube to the autoclave and heated at 90 °C for 15 min. The autoclave was then cooled to RT and the reaction mixture was transferred to a flask. The reaction mixture was analyzed by GS–MS and GC-FID to determine conversion.
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.
References
Kudo, F., Miyanaga, A. & Eguchi, T. Biosynthesis of natural products containing β-amino acids. Nat. Prod. Rep. 31, 1056–1073 (2014).
Lelais, G. & Seebach, D. β2-Amino acids - syntheses, occurrence in natural products, and components of β-peptides1,2. Biopolymer 76, 206–243 (2004). Wiley Periodicals, Inc.
Cheng, R. P., Gellman, S. H. & DeGrado, W. F. β-peptides: from structure to function. Chem. Rev. 101, 3219–3232 (2001).
Alcaide, B., Almendros, P. & Aragoncillo, C. β-lactams: Versatile building blocks for the stereoselective synthesis of non-β-lactam products. Chem. Rev. 107, 4437–4492 (2007).
Hughes, A. B. Amino Acids, Peptides and Proteins in Organic Chemistry. Building (Wiley-VCH Verlag GmbH & Co. KGaA, 2011).
Pollack, M. A. Growth effects of α-methyl homologs of pantothenic acid and β-alanine. J. Am. Chem. Soc. 65, 1335–1339 (1943).
Gardner, P. & Brandon, R. Preparation and reactions of some arylalkyl cyanoacetic esters. J. Org. Chem. 22, 1704–1705 (1957).
Mannich, C. & Ganz, E. β-Aminodicarboxylic acids and aminopolycarboxylic acids. Ber. der Dtsch. Chem. Ges. 55B, 3486–3504 (1922).
Naoya, O. & Tomohiko, A. The Reaction of amino alcohols with acrylates. Bull. Chem. Soc. Jpn 39, 1486–1490 (1966).
Dunn, P. J., Wells, A. S. & Williams, M. T. Green Chemistry in the Pharmaceutical Industry (Wiley-VCH Verlag GmbH & Co. KGaA, 2010).
Mika, L. T., Cséfalvay, E. & Németh, Á. Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability. Chem. Rev. 118, 505–613 (2018).
Brar, S. K., Sarma, S. J. & Pakshirajan, K. 3-Hydroxypropionic Acid In Platform Chemical Biorefinery: Future Green Industry (Elsevier: Amsterdam, 2016).
Werpy, T. & Petersen, G. Top value added chemicals from biomass: Volume I - results of screening for potential candidates from sugar and synthesis gas. in Report no. DOE/GO-102004-1992 (National Renewable Energy Lab., Golden, CO, 2004).
Della Pina, C., Falletta, E. & Rossi, M. A green approach to chemical building blocks. case 3-hydroxypropanoic acid. Green. Chem. 13, 1624–1632 (2011).
Karp, E. M. et al. Renewable acrylonitrile production. Science 358, 1307–1310 (2017).
Kumar, V., Ashok, S. & Park, S. Recent advances in biological production of 3-hydroxypropionic acid. Biotechnol. Adv. 31, 945–961 (2013).
Matsakas, L., Topakas, E. & Christakopoulos, P. New trends in microbial production of 3-hydroxypropionic acid. Curr. Biochem. Eng. 1, 141–154 (2014).
Sheldon, R. A., Poliakoff, M., Perez, E., Tuck, C. O. & Horvath, I. T. Valorization of biomass: deriving more value from waste. Science 337, 695–699 (2012).
Len, C. & Luque, R. Continuous flow transformations of glycerol to valuable products: an overview. Sustain. Chem. Process. 2, 1–10 (2014).
Bähn, S. et al. The catalytic amination of alcohols. ChemCatChem 3, 1853–1864 (2011).
Corma, A., Navas, J. & Sabater, M. J. Advances in one-pot synthesis through borrowing hydrogen catalysis. Chem. Rev. 118, 1410–1459 (2018).
Reed-Berendt, B. G., Polidano, K. & Morrill, L. C. Biomolecular Chemistry hydrogen catalysis using earth-abundant first row transition metals. Org. Biomol. Chem. 17, 1595–1607 (2019).
Wei, D. & Darcel, C. Iron catalysis in reduction and hydrometalation reactions. Chem. Rev. 119, 2550–2610 (2019).
Yan, T., Feringa, B. L. & Barta, K. Iron catalysed direct alkylation of amines with alcohols. Nat. Commun. 5, 5602 (2014).
Zhang, G., Yin, Z. & Zheng, S. Cobalt-catalyzed N‑alkylation of amines with alcohols. Org. Lett. 18, 300–303 (2016).
Elangovan, S. et al. Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat. Commun. 7, 12641 (2016).
Vellakkaran, M., Singh, K. & Banerjee, D. An Efficient and selective nickel-catalyzed direct N-alkylation of anilines with alcohols. ACS Catal. 7, 8152–8158 (2017).
Deng, W. et al. Catalytic amino acid production from biomass-derived intermediates. Proc. Natl Acad. Sci. USA 115, 5093–5098 (2018).
Zhang, M., Imm, S., Bähn, S., Neumann, H. & Beller, M. Synthesis of α-amino acid amides: Ruthenium-catalyzed amination of α-hydroxy amides. Angew. Chem. Int. Ed. 50, 11197–11201 (2011).
Yang, W., Fu, H., Song, Q., Zhang, M. & Ding, Y. Amidate iridium(III) bis(2-pyridyl)phenyl complexes: application examples of amidate ancillary ligands in iridium(III)-cyclometalated complexes. Organometallics 30, 77–83 (2011).
Zhang, Z., Leitch, D. C., Lu, M., Patrick, B. O. & Schafer, L. L. An Easy-to-use, regioselective, and robust bis(amidate) titanium hydroamination precatalyst: Mechanistic and synthetic investigations toward the preparation of tetrahydroisoquinolines and benzoquinolizine alkaloids. Chem. A Eur. J. 13, 2012–2022 (2007).
Haas, K. & Beck, W. Formation of peptides at half-sandwich complexes using β- and γ-amino acid esters and a facile dipeptide synthesis from an N,O-glycinato half sandwich complex [RuCl(NH2CH2CO2)(η6-C6Me6)]. Eur. J. Inorg. Chem. 2001, 2485–2488 (2001).
Yan, T., Feringa, B. L. & Barta, K. Direct N-alkylation of unprotected amino acids with alcohols. Sci. Adv. 3, eaao6494 (2017).
Ding, Z., Chen, F., Qin, J., He, Y. & Fan, Q. Asymmetric hydrogenation of 2,4-disubstituted 1,5-benzodiazepines using cationic ruthenium diamine catalysts: An Unusual achiral counteranion induced reversal of enantioselectivity. Angew. Chem. Int. Ed. 51, 5706–5710 (2012).
Li, C., Wang, C., Villa-Marcos, B. & Xiao, J. Chiral counteranion-aided asymmetric hydrogenation of acyclic imines. J. Am. Chem. Soc. 130, 14450–14451 (2008).
Zhou, S., Fleischer, S., Junge, K. & Beller, M. Cooperative transition-metal and chiral Brønsted acid catalysis: enantioselective hydrogenation of imines to form amines. Angew. Chem. Int. Ed. 50, 5120–5124 (2011).
Li, C., Villa-Marcos, B. & Xiao, J. Metal - Brønsted acid cooperative catalysis for asymmetric reductive amination. J. Am. Chem. Soc. 131, 6967–6969 (2009).
Zhang, Y. et al. Catalytic enantioselective amination of alcohols by the use of borrowing hydrogen methodology: cooperative catalysis by iridium and a chiral phosphoric acid. Angew. Chem. Int. Ed. 53, 1399–1403 (2014).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: History and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Lv, J. & Luo, S. Asymmetric binary acid catalysis: chiral phosphoric acid as dual ligand and acid. Chem. Commun. 49, 847–858 (2013).
Noyori, R. & Ohkuma, T. Asymmetric catalysis by architectural and functional molecular engineering: practical chemo- and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 40, 40–73 (2001).
Kampen, D., Reisinger, C. M. & List, B. Chiral Brønsted acids for asymmetric organocatalysis. in Asymmetric Organocatalysis. Topics in Current Chemistry (ed. List, B.) 291, 395–456 (Springer, Berlin, Heidelberg, 2010).
Banerjee, D., Junge, K. & Beller, M. Cooperative catalysis by palladium and a chiral phosphoric acid: enantioselective amination of racemic allylic alcohols. Angew. Chem. Int. Ed. 53, 13049–13053 (2014).
Thomas, K. D., Adhikari, A. V. & Shetty, N. S. Design, synthesis and antimicrobial activities of some new quinoline derivatives carrying 1,2,3-triazole moiety. Eur. J. Med. Chem. 45, 3803–3810 (2010).
Wang, H. et al. A facile synthesis of 4-gem-difluoromethylene β-lactam and its derivatives from BrCF2CF2Br. J. Fluor. Chem. 127, 1195–1203 (2006).
Acknowledgements
K.B. thanks the European Research Council, ERC Starting Grant 2015 (CatASus) 638076. This work is part of the research programme Talent Scheme (Vidi) with project number 723.015.005 (for K.B.), which is partly financed by the Netherlands Organization for Scientific Research (NWO). A.A. thanks Bálint Fridrich for help with hydrogenation experiments.
Author information
Authors and Affiliations
Contributions
A.A. conducted and designed the experiments, collected and analyzed the data and wrote the manuscript draft. T.Y. conceived the catalyst system, performed exploratory studies and commented on the manuscript. (A.A. and T.Y. contributed equally) K.B. designed experiments, analyzed the data, wrote the manuscript and supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afanasenko, A., Yan, T. & Barta, K. Amination of β-hydroxyl acid esters via cooperative catalysis enables access to bio-based β-amino acid esters. Commun Chem 2, 127 (2019). https://doi.org/10.1038/s42004-019-0229-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-019-0229-x
This article is cited by
-
Visible-light-driven amino acids production from biomass-based feedstocks over ultrathin CdS nanosheets
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.