Abstract
Metal-substituted zeolites are an important type of solid Lewis acid with a wide range of applications. Despite the importance of this type of catalyst, identifying active sites can be challenging because different types of metal sites experience similar environments in zeolites. Here we show direct observation of different tin sites in Sn-β zeolite. Two types of open tin sites are unambiguously identified via correlating the hydroxyl groups to Sn atoms with one- and two-dimensional proton-detected 1H/119Sn correlation solid-state NMR spectroscopy, which only amounts to ca. 17% of the total tin content. A reversible transformation between the open and closed tin site is observed. The results provide valuable insights into the nature of tin sites in Sn-β zeolite and open an avenue for the use of proton-detected solid-state NMR methods for characterization of metal sites in zeolite catalysts.
Similar content being viewed by others
Introduction
Zeolites are among the most important heterogeneous catalysts in the modern chemical and petrochemical industries. Metals are often introduced into zeolites to alter their acidity and thus their catalytic activity. The formation of Lewis acid sites by metals such as tin, titanium, and zirconium in zeolites leads to distinct activities in many important industrial reactions1,2,3,4,5,6,7. Tin-substituted Sn-β zeolite represents a breakthrough in the exploitation of atom-efficient solid Lewis acid catalyst for green and sustainable production of chemicals and fuels2 because of its unparalleled catalytic performance in transformation of biomass and biomass-derived feedstocks7,8,9,10,11,12,13,14,15,16 such as isomerization of glucose8,9 and conversion of sugars to lactic acid7 and furan derivatives11 as well as in the important reactions of Baeyer−Villiger oxidations2 and Meerwein−Ponndor−Verley reduction3. Due to the great potential of the Sn-β zeolite for upgrading renewable energy sources, considerable efforts have been devoted to the synthesis and characterization of Sn-β zeolites17,18,19,20,21,22.
Hydrothermal2 and postsynthetic19,20,21,22,23 strategies have been developed to introduce Sn into β zeolite. It is generally accepted that isomorphously substituted Sn (IV) sites are responsible for the high activity of Sn-β zeolite14,24. However, the similar coordination environment of different Sn sites results in great difficulties in their spectroscopic discrimination. Different techniques have been attempted for the detection of Sn sites in Sn-β17,18,19. Infrared (IR) spectroscopy of probe molecules in combination with theoretical calculations suggests the formation of so-called open (e.g., (SiO)3Sn–OH) and closed (e.g., (SiO)4Sn) Sn sites on hydrothermally synthesized Sn-β25,26. For the open site, the defect-open and hydrolyzed-open configurations were further proposed27, both of them contain the Sn–OH moieties, although the open structure with outer-sphere coordination of SiO− and coordinately unsaturated Sn forming frustrated Lewis pair was also hypothesized to be present on Sn-β28. Solid-state NMR is a powerful tool for the characterization of the active site in zeolites. However, the low concentration of Sn loading (usually lower than 2 wt%) and low isotope abundance of NMR active 119Sn nucleus (8.6%) is an obstacle for NMR detection and reasonable analysis of the obtained signals in low signal-to-noise ratio. Nevertheless, octahedral and tetrahedral Sn sites were differentiated based on the chemical shift of 119Sn on the hydrated and dehydrated Sn-β with isotopically enriched 119Sn atoms2,12,29,30. In order to enhance the detecting sensitivity, the dynamic nuclear polarization surface-enhanced NMR spectroscopy (DNP-SENS) technique27,31,32,33 was recently applied on Sn-β zeolites. With the help of DFT calculations, hydrated closed and open Sn sites were proposed in postsynthetic Sn-β32, while the Sn species was claimed to exist mainly in closed state in Sn-β zeolite synthesized by the direct hydrothermal method27. Since water molecules or additional solvent and radicals used for DNP experiments could be adsorbed on the two types of Sn sites acting as the proton sources to enhance the 119Sn signal27,31,32,33, the Sn sites with and without associated hydroxyl group were unable to be discriminated from 119Sn NMR signal alone. In addition, probably due to its low content and interference from the large quantity of silanols of zeolite, the open site with Sn–OH group in Sn-β is difficult to be directly identified by 1H NMR or IR.
With respective to activity of the Sn sites, the hydroxyl group associated open one was proved to be the active site in the reactions such as Baeyer–Villiger oxidation of cyclohexanone25 and glucose isomerization26,34,35. Additionally, the high activity of the open site was also theoretically suggested by DFT calculations based on cluster models36. However, by far, no experimental technique is available to unambiguously identify the active open Sn site. Besides, the relationship between the open and closed Sn states still remains elusive. Rational design of Sn-containing zeolites with higher activity and selectivity can only be achieved by fully understanding the structure and nature of their active sites.
Here we show that proton-detected 1H/119Sn double-resonance correlation solid-state NMR spectroscopy can be used to unambiguously characterize the Sn active sites in Sn-β zeolites. Two types of open Sn sites containing Sn–OH groups are directly identified by one-dimensional (1D) and two-dimensional (2D) 1H{119Sn} dipolar-mediated (D-) HMQC NMR spectroscopy at high field and fast MAS speed, which only amounts to ca. 17% of the total Sn sites. In combination with 2D 1H {29Si} D-HMQC NMR experiment, a reversible transformation between the open and closed Sn sites is ascertained.
Results
Probing tin sites and Sn–OH groups
A Sn-β zeolite containing 1.2 wt.% of Sn was prepared by the direct hydrothermal method2,37 (denoted as Sn-β). In order to enhance NMR sensitivity, an isotope 119Sn (119Sn, 97.4%)-enriched Sn-β zeolite was synthesized and denoted as 119Sn-β. For comparison, a pure silica β zeolite was also synthesized and denoted as Si-β. Scanning electron microscopy-energy dispersive spectrometer (TEM-EDS), X-ray powder diffraction (XRD), 29Si MAS NMR and diffuse reflectance ultraviolet-visible (DR-UV-vis) analyses showed that all the obtained samples were well crystallized with the topological structure of zeolite β and the metal Sn atoms were homogeneously incorporated into the framework of zeolite β (Supplementary Figs 1–4). 119Sn MAS NMR experiments were performed to study the coordination states of framework Sn sites on 119Sn-β. As shown in Supplementary Fig. 5, there are three 6-coordinated framework Sn sites, and they can be transformed into 4-coordinated Sn sites after dehydration at a temperature above 393 K. Either closed or open Sn sites could contribute to the observed 119Sn signals from the 4-coordinated Sn atoms in the framework of β zeolite27. No signal is visible in the 1H-119Sn CP MAS NMR spectrum of the dehydrated 119Sn-β even with 80 h of acquisition (Supplementary Fig. 5 c and d). This is due to the dramatically reduced cross-polarization efficiency from 1H to 119Sn spins caused by the absence of adsorbed water. The open Sn site featured by the bound hydroxyl group cannot be readily detected by the 1H-119Sn CP MAS NMR, most likely because of its low concentration.
Owing to the high sensitivity of 1H nuclei, 1H MAS NMR experiments at high field (18.8 T) were conducted on the 119Sn-β zeolites (Fig. 1a–e). In order to probe the spatial proximity/interaction between 1H and 119Sn atoms, 1D proton-detected38,39 1H {119Sn} D-HMQC MAS NMR experiments were also carried out at a MAS speed of 40 kHz (Fig. 1f–k). Indeed, we also tried to perform J-HMQC experiments40,41; however, the small J-couplings and fast relaxations in the zeolites hinder their practical implementation on our samples. As shown in Fig. 1a, a main signal at 4.1 ppm and three weak 1H signals at 0.8, 1.2 and 5.4 ppm are observable in hydrated 119Sn-β. The signals at 0.8 and 1.2 ppm come from a trace of residual template, which was confirmed by comparing with those of uncalcined 119Sn-β. After the sample being dehydrated at room temperature (Fig. 1b), the 4.1 ppm signal shifts to high field (3.9 ppm) and is attenuated remarkably, while there is no obvious change on the 5.4 ppm signal, indicating that the two signals probably belong to two types of adsorbed water molecules. Note that only the signal at 5.4 ppm remains in the 1D 1H {119Sn} D-HMQC MAS NMR spectrum (Fig. 1f, h) in which only the protons interacting with Sn species can be observable, suggesting that this 1H signal is associated with water molecules chemically adsorbed on Sn sites. In contrast, the strong signals at 4.1 and 3.9 ppm are completely suppressed, indicative of the absence of Sn atoms in close proximity. Thus, we can assign these two signals to physically adsorbed water molecules in zeolite channels. When the dehydration temperature is increased to 393 K, new signals appear at 2.5, 2.1, and 1.8 ppm in the 1H MAS NMR spectrum (Fig. 1c). The three signals can be assigned to different kinds of non-hydrogen-bonded Si–OH species while a broad signal at 3.6 ppm may come from hydrogen-bonded SiOH groups42,43. They disappeared in the corresponding HMQC spectrum (Fig. 1i). Indeed, the formation of Si–OH species is confirmed by 1H-29Si CP MAS NMR experiments (Supplementary Fig. 3). It is interesting to note that a weak broad signal at 0.3 ppm is visible in the 1H MAS NMR spectrum (Fig. 1c), which produces two well-resolved peaks at ~0.4 and ~0.2 ppm in the corresponding 1D 1H {119Sn} HMQC spectrum (Fig. 1i). Considering the 1H-119Sn CP NMR result together, the observation allows us to conclude that two Sn–OH groups are probably present on the dehydrated 119Sn-β. When 119Sn-β zeolite was dehydrated at 673 K, the two Sn–OH signals at 0.4 and 0.2 ppm completely disappear in the 1D 1H{119Sn} HMQC spectrum (Fig. 1j), which is accompanied by a slight decline of the Si–OH signal at 2.1 ppm in the 1H MAS NMR spectrum (Fig. 1d) as compared with that at 393 K (Fig. 1c). After the 673 K dehydrated sample was rehydrated (exposed in air moisture for 30 days) and then dehydrated at 393 K, the two signals at 0.2 and 0.4 ppm appear again (Fig. 1k) accompanied with the recovery of the Si–OH signal at 2.1 ppm (Fig. 1e). This indicates a reversible formation of Sn–OH groups in Sn-β. Note that Sn–OH species is unobservable in the 1D 1H {119Sn} HMQC spectra when the chemically adsorbed water (5.4 ppm) is not removed (Fig. 1f, h). Since such water molecules are most likely adsorbed on framework Sn atoms forming 6-coordinated Sn sites, we suspect that the relatively large amount of water molecules and the low concentration of Sn–OH species make the Sn–OH undetectable.
Connectivity between tin atoms and hydroxyl groups
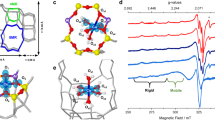
To get insight into the local structure of Sn sites in 119Sn-β, 2D 1H {119Sn} D-HMQC spectra were recorded (see Fig. 2). The spectra with full chemical shift range of 1H and 119Sn are shown in Supplementary Fig. 6. Both hydrated 119Sn-β and the corresponding sample dehydrated at 298 K exhibit a similar 1H–119Sn correlation peak at (5.4, −686) ppm (Fig. 2a, b), revealing the presence of water molecule-bound 6-coordinated Sn sites. This is also in agreement with the 1H-119Sn CP MAS NMR result that the 119Sn signal at −686 ppm is selectivity enhanced by the neighboring protons from chemically adsorbed water molecules (Supplementary Fig. 5a). For 119Sn-Beta dehydrated at 393 K, four 1H-119Sn correlation peaks are observable in the HMQC spectrum (Fig. 2c). The two 4-coordinated Sn sites at −443 and −429 ppm are correlated to 1H species at 0.1–0.4 ppm. Further analysis shows that the two 1H signals at 0.37 and 0.20 ppm have correlations with the Sn signal at −443 ppm while the two 1H signals at 0.34 and 0.17 ppm exhibit correlations with the Sn signal at −429 ppm (Fig. 2c). It is interesting to note that when 119Sn decoupling was applied during 1H acquisition in the D-HMQC experiments, the four correlation peaks merge into two ones centered at (0.28, −443) and (0.26, −429) ppm (Fig. 2d). Therefore, the four resolved correlation peaks in Fig. 2c should be due to the doublet splitting of two types of 1H signals caused by J-coupling between spin pairs of 1H and 119Sn. We further measured the J-coupling constant from the peak distance between the doublet fine structure, which was determined to be ~136 Hz, in consistent with the 2J (119Sn–1H) constant of 130 Hz for Sn–OH species in monoalkyl-SnCl3–x(OH) x solution44,45. These results provide strong evidence that there are two types of Sn–OH groups; one corresponds to the Sn atom at −443 ppm bound to the hydroxyl group at 0.28 ppm, and the other is the Sn atom at −429 ppm bound to the hydroxyl group at 0.26 ppm. They can be attributed to two types of open Sn sites ((SiO)3Sn–OH) located at different T sites on the framework of β zeolite. This is in agreement with the recent DFT study, which indicated that there are two T sites to stabilize the open Sn sites on β zeolite46. Here the two types of open Sn sites were directly observed under dehydration condition without addition of solvent and radicals, and the influences of water, solvent, and radicals can be excluded. To the best of our knowledge, this is the first time to unambiguously identify two types of open Sn sites on Sn-β zeolite, which shows different NMR characteristics in the proton-detected 1H{119Sn} double-resonance correlation spectra. The direct observation of the Sn–OH groups indicates that either defect- or hydrolyzed-open sites are present, although they are formed differently on the sample27.
Identification of open Sn sites by proton-detected 1H/119Sn correlation NMR. Two-dimensional 1H {119Sn} HMQC MAS NMR spectra of 119Sn-β a without dehydration, b dehydrated at 298 K, c dehydrated at 393 K without 119Sn decoupling, and d dehydrated at 393 K with 119Sn decoupling. Projections of 1H and 119Sn dimensions are shown in black. Representative slices along −429 ppm (red) and −443 ppm (blue) in the F1 dimension are also displayed in c and d
On the basis of 1H {119Sn} D-HMQC NMR results, the 119Sn MAS NMR spectra of samples dehydrated at 393 and 673 K were deconvoluted (Fig. 3): the signals at −443 and −429 ppm correspond to the open sites and the signals at −446, −441 and −422 ppm come from the closed Sn sites. Accordingly, the content of two types of Sn sites could be estimated. As listed in Table 1, the open Sn sites is ca. 17% of the total Sn sites on the sample dehydrated at 393 K, in consistent with that (ca. 20%) previously determined by site titration with IR adsorption of CD3CN25. Higher temperature dehydration (673 K) leads to the drop of the open sites to zero with the generation of closed sites due to the dehydroxylation of the Sn–OH groups47.
Quantitative 119Sn solid-state NMR analysis of Sn sites in Sn-β. Deconvolution of 119Sn MAS NMR spectra of 119Sn-β zeolites dehydrated at 393 K (a) and 673 K (b). Solid lines show experimental (black) and simulated (green) spectra, and dash lines show the individual components of closed (blue) and open (red) Sn sites
Interconversion of open and closed tin sites
The transformation of the open site into the closed one could be responsible for the disappearance of NMR signals of Sn–OH groups in the 1D 1H {119Sn} D-HMQC MAS spectrum (Fig. 1j) during the dehydration process. In order to gain more insight into this process, 2D 1H {29Si} D-HMQC NMR experiments were performed on 119Sn-Beta with different degrees of dehydration (Fig. 4). For the sample dehydrated at 393 K (Fig. 4a), the appearance of correlation peaks between 1H signals at 1.8, 2.1, 2.5, and 3.6 ppm and 29Si signal at −102 ppm indicates the presence of different types of Si–OH groups (Q3 sites, Si(OSi)3OH). It is noteworthy that the correlation peak of Q3 site at (2.1, −102) ppm is evidently reduced when the sample is dehydrated at 673 K (Fig. 4b), indicating its low stability in the dehydroxylation process. This is also confirmed by comparing the Q3 sites on the representative slices along 1H signals at 1.8, 2.1, and 2.5 ppm (Supplementary Fig. 7). These Q3 sites could be involved in dehydration with (SiO)3Sn–OH species, leading to the transformation of open Sn site to closed one ((SiO)4Sn). When the sample is rehydrated, the closed Sn site could be hydrolyzed to regenerate an open Sn site which is visible after the sample is dehydrated at 393 K (Fig. 1k).
Taking all the results together, the Sn active sites and their evolutions observed on Sn-β zeolite can be pictured (Fig. 5). Both closed and open Sn sites are present and there is a reversible conversion between them. The defect-open or hydrolyzed-open sites are not diffrentiated here. In hydrated sample, two water molecules are chemically adsorbed on the same Sn atom, forming a 6-coordinated open Sn site (Fig. 5a). After the removal of the chemically adsorbed water molecules by dehydration at 393 K, a 4-coordinated open Sn site is isolated (Fig. 5b). Further increasing the dehydration temperature to 673 K causes the removal of water between the 4-coordinated open Sn site and the neighboring Si–OH group, leading to the formation of a 4-coordinated closed Sn site (Fig. 5c). This is responsible for the disappearance of the open Sn site and the only presence of the closed one on the highly dehydrated Sn-β. However, the closed Sn site shows reactivity to water molecules even at room temperature. The Sn–O–Si bond of the closed Sn site can be broken by the attack of water molecules, through which the closed Sn site is reversely transformed into an open one by forming the Sn–OH group47. Note that the recovery of open Sn site from closed one is a slow process (in air moisture for 30 days) at room temperature. We found that a quick saturation adsorption of water (in 24 h) onto the 119Sn-β zeolite dehydrated at 673 K in a Petri dish did not lead to the generation of open Sn sites (Supplementary Fig. 8). Instead, a moderate heating of the hydrated sample at 393 K in air facilitates their formation.
Discussion
In summary, open Sn sites containing Sn–OH groups in Sn-β are unambiguously identified and quantified. 2D 1H {119Sn} D-HMQC MAS NMR spectroscopy enables differentiation of two types of open Sn sites by correlating the hydroxyl groups and associated Sn species. Both open and closed Sn sites are present and the transformation between them is reversible on Sn-β zeolite. This information provides valuable insights into the nature of active Sn sites in Sn-β zeolite. Hydroxyl groups containing metal species in zeolites such as TS-1 are often thought to be the active sites48,49,50, which however are hardly identified due to the low concentration and similar environment to coexisting metal sites. Our results have important implications for the characterization of active metal sites in zeolites in particular for those with different metal speciation, which is essential for fine-tuning and rational design of catalysts.
Methods
Sample preparation
Sn-β zeolites were synthesized by direct hydrothermal method in fluoride medium2. Typically, 8.5 g of tetraethyl orthosilicate (TEOS; Sinopharm Chemical Reagent Co., Ltd, 99%) was hydrolyzed in 12.7 g aqueous solution of 25% tetraethylammonium hydroxide (TEAOH; J&K Scientific) under stirring. Then a solution containing 0.09 g SnCl4·5H2O (Sinopharm Chemical Reagent Co., Ltd, 99%) was added, and the mixture was stirred until the ethanol formed upon hydrolysis of TEOS was evaporated. About 1.2 mL 40% HF (Sinopharm Chemical Reagent Co., Ltd, 98%) was added to the obtained clear solution (ca. 12 g), and a thick paste was formed. Then, an aqueous suspension of 0.10 g Si-β seeds was added. For Si-β seeds, commercial Al-β was treated with 70% nitric acid (Sinopharm Chemical Reagent Co., Ltd, 98%) followed by filtration and drying, and then the dealuminated Si-β was obtained as seeds. The mixture containing Si-β seeds was evaporated in the fume hood, resulting in a final gel with the composition (mole ratio) of: 1.0 SiO2:0.0062 Sn:0.55 TEAOH:7.5 H2O:0.55 HF. Then, the ca. 10.50 g of mixture was transferred into an 18 mL Teflon-lined stainless-steel autoclave was placed in a preheated oven and kept at 413 K for 30 days in static state. Finally, the solid product was recovered by filtration and washing. Typically, 1.00 g sample was washed by 100 mL deionized water for more than three times, until the Cl− ions was completely removed. The sample was then dried at 353 K overnight followed by calcination at 873 K for 10 h. For calcination, 0.30 g sample was placed in a 1.5 cm × 4.0 cm quartz boat and calcined at static state air condition with a temperature program of 3 K/min to 873 K, then holding 10 h at 873 K. The isotope 119Sn-enriched 119Sn-β zeolite was prepared by a similar route, using a solution of 119Sn precursor37, which was prepared by dissolving of 0.03 g 119Sn metal foil (ISOFLEX, USA, 119Sn abundance 97.4%) into 0.2 mL aqueous solution with an appropriate amount of 37% hydrochloric acid (Sinopharm Chemical Reagent Co., Ltd, 98%) and 30 % hydrogen peroxide (Sinopharm Chemical Reagent Co., Ltd, 98%) at 323 K. After the hydrochloric acid solution was evaporated at 393 K, the obtained white solid was dissolved into 1 mL water. The Si-β zeolite was synthesized with Si-β seeds by the hydrothermal method in fluoride media without adding any Sn precursor.
Calcined 119Sn-β zeolites were dehydrated at 298, 393, and 673 K with a pressure of 10−3 Pa on a vacuum line over a period of 2 h.
SEM-EDS experiments
SEM-EDS experiments were performed on a Hitachi FE-SEM SU8010 field-emission scanning electron microscope; the accelerating voltage was operated at 5 kV.
DR-UV-vis experiments
DR-UV-vis spectra were collected on an Agilent Cary 4000 UV-Vis spectrometer at room temperature. The scan rate is 2 nm/s for DR-UV-vis measurements.
XRD experiments
XRD patterns were recorded on a Panalytical X’ Pert PRO X-ray diffractometer (40 kV, 40 mA) using CuKα (λ = 1.5406 Å) radiation. The scan rate is 0.05° per second for XRD measurements.
Solid-state NMR experiments
29Si MAS NMR and 1H-29Si CP MAS NMR experiments were carried out at 7.05 T on a Varian Infinity plus-300 spectrometer with a 7.5 mm double-resonance probe. The resonance frequencies were 299.78 and 59.55 MHz for 1H and 29Si, respectively. Single-pulse 29Si MAS experiments with 1H decoupling were performed by using a π/2 pulse width of 6.2 μs and a repetition time of 60 s. 1H-29Si CP MAS experiments were performed by using with a contact time of 4 ms and with a repetition time of 2 s. The magic angle spinning rate was set to 4 kHz. The 29Si chemical shift was referenced to kaoline at −91.0 ppm, as the second reference to tetramethylsilane.
119Sn MAS NMR and 1H-119Sn CP MAS NMR experiments were carried out at 7.05 T on a Varian Infinity plus-300 spectrometer with a 4 mm double-resonance probe. The resonance frequencies were 299.78 MHz and 111.72 MHz for 1H and 119Sn, respectively. Single-pulse excitation 119Sn MAS experiments were performed on the zeolite samples by using a π/2 pulse width of 4.6 μs, a repetition time of 200 s, and a magic angle spinning rate of 12 kHz. 1H-119Sn CP MAS experiments were performed by using a contact time of 4 ms and a repetition time of 20 s. The 119Sn chemical shift was referenced to tetracyclohexyltin at −97.4 ppm. Each spectrum was accumulated for ca. 80 h.
1H MAS, 1H {119Sn} D-HQMC and 1H {29Si} D-HQMC NMR experiments were carried out at 18.8 T on a Bruker AvanceШ 800 spectrometer, using a 1.9 mm HX double-resonance probe at a spinning rate of 40 kHz. The resonance frequencies were 800.36, 298.33, and 158.99 MHz for 1H, 119Sn, and 29Si, respectively. 1H MAS NMR experiments were performed on the zeolite samples by using the Hahn-echo pulse sequence (π/2–τ–π–τ –acquisition) with a π/2 pulse width of 2.0 μs and a repetition time of 20 s, where τ equals to three rotor period (75 μs).
The pulse sequence for 1H {119Sn/29Si} D-HMQC experiments is illustrated in Supplementary Fig. 9. The rf field strength for the 1H π/2 and π pulses was set to 125 kHz. The pulse lengths for π/2 pulses on the 119Sn channel or 29Si channel were fixed to 3.3 or 3.7 μs, respectively. SR4 recoupling on the 1H channel was used with νnut,1H = 80 kHz, the total recoupling time τ was set to 1200 and 2000 μs for 1H {119Sn} and 1H {29Si} D-HMQC experiments, respectively. The fast MAS and short recoupling time makeit possible to mainly detect the correlations corresponding to the stronger interactions between 1H and 119Sn atoms in 2D 1H {119Sn} D-HMQC experiments. The low-power continuous-wave 119Sn decoupling, with an amplitude of ~3 kHz, was used during the 1H acquisition in the Fig. 2d and Supplementary Fig. 6d. Except for these two spectra, no decoupling was applied during the acquisition of the 2D D-HMQC spectra. The increment interval in the indirect dimension was set to 25 μs. Typically, 2D 1H {119Sn} D-HMQC spectra were acquired using 30 increments and 320 scans, and 2D 1H {29Si} D-HMQC spectra were acquired using 40 increments and 240 scans. The recycle delay was set to 8 s.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Huybrechts, D. R. C., Debruycker, L. & Jacobs, P. A. Oxyfunctionalization of alkanes with hydrogen peroxide on titanium silicalite. Nature 345, 240–242 (1990).
Corma, A., Nemeth, L. T., Renz, M. & Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations. Nature 412, 423–425 (2001).
Corma, A., Domine, M. E., Nemeth, L. & Valencia, S. Al-free Sn-beta zeolite as a catalyst for the selective reduction of carbonyl compounds (Meerwein−Ponndorf−Verley reaction). J. Am. Chem. Soc. 124, 3194–3195 (2002).
Sushkevich, V. L., Ivanova, I. I., Tolborg, S. & Taarning, E. Meerwein-Ponndorf-Verley-Oppenauer reaction of crotonaldehyde with ethanol over Zr-containing catalysts. J. Catal. 316, 121–129 (2014).
Van de Vyver, S. & Román-Leshkov, Y. Metalloenzyme-like zeolites as Lewis acid catalysts for C-C bond formation. Angew. Chem. Int. Ed. 54, 12554–12561 (2015).
Palagin, D., Sushkevich, V. L., Ivanova, I. I. & Coupling, C.-C. Catalyzed by zeolites: is enolization the only possible pathway for aldol condensation? J. Phys. Chem. C 120, 23566–23575 (2016).
Holm, M. S., Saravanamurugan, S. & Taarning, E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 328, 602–605 (2010).
Moliner, M., Román-Leshkov, Y. & Davis, M. E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 107, 6164–6168 (2010).
Román-Leshkov, Y., Moliner, M., Labinger, J. A. & Davis, M. E. Mechanism of glucose isomerization using a solid Lewis acid catalyst in water. Angew. Chem. Int. Ed. 49, 8954–8957 (2010).
Taarning, E. et al. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ. Sci. 4, 793–804 (2011).
Nikolla, E., Román-Leshkov, Y., Moliner, M. & Davis, M. E. “One-Pot” synthesis of 5-(hydroxymethyl)furfural from carbohydrates using tin-beta zeolite. ACS Catal. 1, 408–410 (2011).
Bermejo-Deval, R. et al. Metalloenzyme-like catalyzed isomerizations of sugars by Lewis acid zeolites. Proc. Natl. Acad. Sci. USA 109, 9727–9732 (2012).
Kubicka, D., Kubickova, I. & Cejka, J. Application of molecular sieves in transformations of biomass and biomass-derived feedstocks. Catal. Rev. 55, 1–78 (2013).
Dapsens, P. Y., Mondelli, C. & Perez-Ramirez, J. Design of Lewis-acid centres in zeolitic matrices for the conversion of renewables. Chem. Soc. Rev. 44, 7025–7043 (2015).
Ennaert, T. et al. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 45, 584–611 (2016).
Luo, H. Y., Lewis, J. D. & Román-Leshkov, Y. Lewis acid zeolites for biomass conversion: perspectives and challenges on reactivity, synthesis, and stability. Annu. Rev. Chem. Biomol. Eng. 7, 663–692 (2016).
Bare, S. R. et al. Uniform catalytic site in Sn-beta-zeolite determined using X-ray absorption fine structure. J. Am. Chem. Soc. 127, 12924–12932 (2005).
Roy, S., Bakhmutsky, K., Mahmoud, E., Lobo, R. F. & Gorte, R. J. Probing Lewis acid sites in Sn-beta zeolite. ACS Catal. 3, 573–580 (2013).
Tang, B. et al. Improved postsynthesis strategy to Sn-beta zeolites as Lewis acid catalysts for the ring-opening hydration of epoxides. ACS Catal. 4, 2801–2810 (2014).
Li, P. et al. Postsynthesis and selective oxidation properties of nanosized Sn-beta zeolite. J. Phys. Chem. C 115, 3663–3670 (2011).
Hammond, C., Conrad, S. & Hermans, I. Simple and scalable preparation of highly active Lewis acidic Sn-beta. Angew. Chem. Int. Ed. 51, 11736–11739 (2012).
Dijkmans, J. et al. Post-synthesis Snβ: an exploration of synthesis parameters and catalysis. J. Catal. 330, 545–557 (2015).
Vega-Vila, J. C., Harris, J. W. & Gounder, R. Controlled insertion of tin atoms into zeolite framework vacancies and consequences for glucose isomerization catalysis. J. Catal. 344, 108–120 (2016).
Corma, A. & Garcia, H. Lewis acids as catalysts in oxidation reactions: from homogeneous to heterogeneous systems. Chem. Rev. 102, 3837–3892 (2002).
Boronat, M., Concepcion, P., Corma, A., Renz, M. & Valencia, S. Determination of the catalytically active oxidation Lewis acid sites in Sn-beta zeolites, and their optimisation by the combination of theoretical and experimental studies. J. Catal. 234, 111–118 (2005).
Harris, J. W. et al. Titration and quantification of open and closed Lewis acid sites in Sn-Beta zeolites that catalyze glucose isomerization. J. Catal. 335, 141–154 (2016).
Wolf, P. et al. Correlating synthetic methods, morphology, atomic-level structure, and catalytic activity of Sn-β catalysts. ACS Catal. 6, 4047–4063 (2016).
Dijkmans, J. et al. An inner-/outer-sphere stabilized Sn active site in β-zeolite: spectroscopic evidence and kinetic consequences. ACS Catal. 6, 31–46 (2016).
Dijkmans, J. et al. Cooperative catalysis for multistep biomass conversion with Sn/Al beta zeolite. ACS Catal. 5, 928–940 (2015).
Bermejo-Deval, R., Gounder, R. & Davis, M. E. Framework and extraframework tin sites in zeolite beta react glucose differently. ACS Catal. 2, 2705–2713 (2012).
Gunther, W. R., Michaelis, V. K., Caporini, M. A., Griffin, R. G. & Román-Leshkov, Y. Dynamic nuclear polarization NMR enables the analysis of Sn-beta zeolite prepared with natural abundance 119Sn precursors. J. Am. Chem. Soc. 136, 6219–6222 (2014).
Wolf, P. et al. NMR signatures of the active sites in Sn-beta zeolite. Angew. Chem. Int. Ed. 53, 10179–10183 (2014).
Wolf, P. et al. Identifying Sn site heterogeneities prevalent among Sn-beta zeolites. Helv. Chim. Acta 99, 916–927 (2016).
Bermejo-Deval, R., Orazov, M., Gounder, R., Hwang, S. J. & Davis, M. E. Active sites in Sn-beta for glucose isomerization to fructose and epimerization to mannose. ACS Catal. 4, 2288–2297 (2014).
Hwang, S.-J. et al. Solid state NMR characterization of Sn-beta zeolites that catalyze glucose isomerization and epimerization. Top. Catal. 58, 435–440 (2015).
Yang, G., Pidko, E. A. & Hensen, E. J. M. The mechanism of glucose isomerization to fructose over Sn-BEA zeolite: a periodic density functional theory study. Chemsuschem 6, 1688–1696 (2013).
Kolyagin, Y. G., Yakimov, A. V., Tolborg, S., Vennestrøm, P. N. R. & Ivanova, I. I. Application of 119Sn CPMG MAS NMR for fast characterization of Sn sites in zeolites with natural 119Sn isotope abundance. J. Phys. Chem. Lett. 7, 1249–1253 (2016).
Wiench, J. W., Bronnimann, C. E., Lin, V. S. Y. & Pruski, M. Chemical shift correlation NMR spectroscopy with indirect detection in fast rotating solids: studies of organically functionalized mesoporous silicas. J. Am. Chem. Soc. 129, 12076–12077 (2007).
Palmer, A. G., Cavanagh, J., Wright, P. E. & Rance, M. Sensitivity improvement in proton-detected two-dimensional heteronuclear correlation NMR spectroscopy. J. Magn. Reson. 93, 151–170 (1991).
Lesage, A., Sakellariou, D., Steuernagel, S. & Emsley, L. Carbon−proton chemical shift correlation in solid-state NMR by through-bond multiple-quantum spectroscopy. J. Am. Chem. Soc. 120, 13194–13201 (1998).
Wang, Q. et al. Signal enhancement of J-HMQC experiments in solid-state NMR involving half-integer quadrupolar nuclei. Chem. Commun. 49, 6653–6655 (2013).
Freude, D., Hunger, M., Pfeifer, H. & Schwieger, W. 1H MAS NMR studies on the acidity of zeolites. Chem. Phys. Lett. 128, 62–66 (1986).
Hunger, M., Ernst, S., Steuernagel, S. & Weitkamp, J. High-field 1H MAS NMR investigations of acidic and non-acidic hydroxyl groups in zeolites H-Beta, H-ZSM-5, H-ZSM-58 and H-MCM-22. Microporous Mater. 6, 349–353 (1996).
Blunden, S. J. & Hill, R. An investigation of the base hydrolysis of methyl- and butyl-tin trichloride in aqueous solution by 1H and 119Sn NMR spectroscopy. Inorg. Chim. Acta 177, 219–223 (1990).
Blunden, S. J., Smith, P. J. & Gillies, D. G. An investigation of the hydrolysis products of monoalkyltin trichlorides by 119mSn Mössbauer, and 1H and 119Sn NMR spectroscopy. Inorg. Chim. Acta 60, 105–109 (1982).
Josephson, T. R., Jenness, G. R., Vlachos, D. G. & Caratzoulas, S. Distribution of open sites in Sn-Beta zeolite. Microporous Mesoporous Mater. 245, 45–50 (2017).
Yakimov, A. V., Kolyagin, Y. G., Tolborg, S., Vennestrøm, P. N. R. & Ivanova, I. I. 119Sn MAS NMR study of the interaction of probe molecules with Sn-BEA: the origin of penta- and hexacoordinated tin formation. J. Phys. Chem. C 120, 28083–28092 (2016).
Sushkevich, V. L., Palagin, D. & Ivanova, I. I. With open arms: open sites of ZrBEA zeolite facilitate selective synthesis of butadiene from ethanol. ACS Catal. 5, 4833–4836 (2015).
Gleeson, D. et al. The architecture of catalytically active centers in titanosilicate (TS-1) and related selective-oxidation catalysts. Phys. Chem. Chem. Phys. 2, 4812–4817 (2000).
Maschmeyer, T., Rey, F., Sankar, G. & Thomas, J. M. Heterogeneous catalysts obtained by grafting metallocene complexes onto mesoporous silica. Nature 378, 159–162 (1995).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 21622311, 21503269, 21573278, 21733013, and 91745111) and key program for frontier science of the Chinese Academy of Sciences (QYZDB-SSW-SLH027).
Author information
Authors and Affiliations
Contributions
G.Q., Q. Wang, C.W., and X.Z. prepared the samples and carried XRD and 119Sn MAS NMR experiments. Q. Wu, X.M., and F.X. performed and analyzed the TEM-EDS and DR-UV-vis spectra. G.Q., Q. Wang, J.X., and F.D. collected and analyzed the high-field 1H MAS NMR and 1H {119Sn} D-HMQC spectra; G.Q., Q. Wang, J.X., and F.D. wrote the manuscript, and all authors discussed the experiments and final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qi, G., Wang, Q., Xu, J. et al. Direct observation of tin sites and their reversible interconversion in zeolites by solid-state NMR spectroscopy. Commun Chem 1, 22 (2018). https://doi.org/10.1038/s42004-018-0023-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-018-0023-1
This article is cited by
-
Hierarchical Fe–Sn/Beta catalyzes the conversion of glucose to methyl lactate
Journal of Porous Materials (2021)
-
Multifunctional heteroatom zeolites: construction and applications
Frontiers of Chemical Science and Engineering (2021)
-
Mapping the oxygen structure of γ-Al2O3 by high-field solid-state NMR spectroscopy
Nature Communications (2020)
-
Solid-state NMR for metal-containing zeolites: From active sites to reaction mechanism
Frontiers of Chemical Science and Engineering (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.