Abstract
Manganese oxide (MnO2) has long been investigated as a pseudo-capacitive material for fabricating fiber-shaped supercapacitors but its poor electrical conductivity and its brittleness are clear drawbacks. Here we electrochemically insert nanostructured MnO2 domains into continuously interconnected carbon nanotube (CNT) networks, thus imparting both electrical conductivity and mechanical durability to MnO2. In particular, we synthesize a fiber-shaped coaxial electrode with a nickel fiber as the current collector (Ni/CNT/MnO2); the thickness of the CNT/MnO2 hybrid nanostructured shell is approximately 150 μm and the electrode displays specific capacitances of 231 mF cm−1. When assembling symmetric devices featuring Ni/CNT/MnO2 coaxial electrodes as cathode and anode together with a 1.0 M Na2SO4 aqueous solution as electrolyte, we find energy densities of 10.97 μWh cm−1. These values indicate that our hybrid systems have clear potential as wearable energy storage and harvesting devices.
Similar content being viewed by others
Introduction
Fiber-shaped supercapacitors (FSCs) constitute a class of one-dimensional electrical devices for energy storage1,2,3. Their wearable nature, low weight, and high flexibility make them suitable for powering portable microelectromechanical devices4,5,6 or other wearable electronic systems7,8,9,10,11. Similar to conventional planar- and cylindrical-shaped supercapacitors, FSCs store energy via either the electrical double layer (EDL) principle or the pseudo-capacitance mechanism1. For the EDL-based FSCs7,10,12,13,14,15,16,17, energy density is governed by the overall capability of the absorbing electrolytes (cations and anions), by having active materials embedded within electrodes. For the pseudo-capacitive FSCs4,5,6,8,9,18,19,20,21, on the other hand, the overall amount of the redox-active materials of the electrodes is key for determining the energy density. Purely EDL-based FSCs with carbon materials arranged as fibers and/or yarns have resulted in high power densities and enhanced cycling stabilities; their energy densities however are often limited to a few mF cm−1 22,23,24,25. Activated carbons, carbon nanotubes (CNTs), graphene, and reduced graphene oxide (rGO) have also been used as active materials for fabricating fiber-shaped and/or yarn-shaped electrodes.
Of the many outstanding achievements, an effort made by Liu et al.26 is noteworthy: they built up a typical supercapacitor exhibiting 110 mF cm−1 energy density, which appears to be the highest value reported to date for purely EDL-based FSCs. The rGO being electrochemically deposited on nickel-coated multifilament cotton yarns was also used as an active material. Its high energy density was attributed to the large surface area of rGO. As current collectors, nickel-coated cotton yarns were synthesized via electroless deposition26, thus preserving high flexibility and the best electrical resistance has been optimized at ca. 1.3 Ω cm−1, a value which is much higher than that of normal nickel fibers (ca. 5.1 × 10−2 Ω cm−1).
For pseudo-capacitive FSCs, energy density values based on reversible redox interactions can be as high as many times that of purely EDL-based capacitors1. Therefore, the loading of the redox-active materials has been a challenge in the fabrication of the pseudo-capacitive FSCs. Redox-active materials, especially inorganic systems such as MnO2, RuO2, Ni(OH)2, and Co(OH)2, are highly brittle and exhibit poor electrical conductivity values27. In this context, Kim and co-workers11,28] have recently reported an effective approach that is able to overcome both the brittleness and low electrical conductivity. In particular, manganese oxide (MnO2) nanoparticles were uniformly deposited on a piece of a CNT sheet via drop casting a dispersion containing MnO2 nanoparticles which was then twisted into bi-scrolled yarns. MnO2 loading rate could be maximized up to 93 wt% while the CNT sheet based yarns retained excellent flexibility and mechanical durability. In this case, the maximum energy density reported was 60.6 mF cm−1. Therefore, wrapping MnO2 with CNT sheet constitutes an alternative to maximize the loading ratio of the redox-active materials. However, the full activation of MnO2 is not possible via physically wrapping the redox-active materials with CNT sheets.
Hybrid structures resembling tissue cells and capillaries in living organisms inspired our studies; one could visualize MnO2 domains as tissue cells and CNT networks as capillaries. In this study we describe the synthesis of CNT/MnO2 hybrid nanostructures by electrochemically inserting nanostructured MnO2 domains into continuously interconnected CNT networks. These CNT/MnO2 hybrid nanostructures are self-conducting, and the transfer of electrons during charge and discharge can be achieved rapidly; this occurs almost independently of the thickness of the CNT/MnO2 system. We also fabricate different coaxial fiber-shaped electrodes using CNT/MnO2 hybrid nanostructures as the pseudo-capacitive materials. The fiber core, which functions as current collectors can be either metal wires/fibers or carbon fibers. As the pseudo-capacitive materials of FSCs, our CNT/MnO2 hybrid nanostructures can be loaded up to a maximum thicknesses of ca. 150 μm, while both the Faradic efficiency and the mechanical properties are retained with excellent performance. In particular, we obtain specific capacitances of 231 mF cm−1, which is about four times the value reported for the CNT-sheet-wrapped MnO2 electrodes11. We believe these results pave the way to establish new assemblies with a high impact in energy-related applications.
Results
Synthesis and characterization of fiber-shaped Ni/CNT/MnO2 electrodes
Figure 1a schematically illustrates the process for fabricating a coaxial fiber-shaped CNT/MnO2 hybrid nanostructured electrode, in which a nickel fiber (O.D. 200 μm) was used as the current collector (denoted as Ni/CNT/MnO2 electrode). Two key features need to be following during preparation: (i) the interconnected CNT networks with thicknesses of ca. 300 nm were prepared by “dipping and drying”, and (ii) nanostructured MnO2 domains were electrochemically deposited within the CNT networks by cyclic voltammetry (CV). The thickness of the MnO2 domains was precisely controlled by controlling CV scan rate. These two steps (step (i) and step (ii)) were repeated for several cycles until the CNT/MnO2 hybrid nanostructures with a desirable thickness were achieved. For example, a Ni/CNT/MnO2 electrode carrying CNT/MnO2 with a total thickness of 9.1 μm was prepared after 9 cycles of MnO2 deposition at a CV scan rate of 50 mV s−1 and 8 cycles for depositing the CNT networks. Energy dispersive spectroscopy (EDS) analysis of carbon (contained in CNTs), manganese, and oxygen (present in MnO2) are shown in Supplementary Figure 1a-c. The data indicated that the CNT/MnO2 hybrid nanostructures are indeed constructed by MnO2 and CNTs. Elemental EDS line scans (Fig. 1b) are overlapped with element mappings of carbon, manganese, and oxygen (Fig. 1c), and scanning electron microscopy (SEM) images (Fig. 1d). These provided a clear composition of the CNT/MnO2 hybrid nanostructures. It is clear that MnO2 domains have penetrated into the CNT networks (Fig. 1e, f). The initial thickness of CNT networks was 300–400 nm but it expanded to 1.1–1.3 μm after the electrochemical deposition of the nanostructured MnO2 domains. In our experiments, manganese acetate dissolved in 0.1 M sodium sulfate aqueous solution was used as the bath for electrodepositing the nanostructured MnO2 domains. In particular, manganese ions (Mn2+) penetrated into the whole CNT networks via self-diffusion. The expansion of the CNT networks from 300–400 nm to 1.1–1.3 μm could be due to the formation of the nanostructured MnO2 nanoparticles within the CNT networks, similar to previous observations5,29. A schematic illustration is shown in Fig. 1e, depicting the distribution of CNT/MnO2 hybrid nanostructures. While MnO2 domains were densely packed, the CNT networks exhibited plenty of entangled cavities (Fig. 1d, f). EDS mapping for sodium (cation of electrolyte, Na2SO4) was also recorded (Supplementary Figure 1d). In this case, sodium ions were uniformly distributed through the CNT/MnO2 hybrid nanostructures, thus indicating a high accessibility of the electrolyte. Supplementary Figure 1e shows an SEM image of a cross-sectional area at low resolution. Neither the physically overwrapping method11 nor the “layer-by-layer” method30 are applicable for depositing MnO2 nanostructures into the heavily interconnected CNT networks we produced with high electrical conductivity values.
Fabrication schemes and images of a CNT/MnO2 hybrid nanostructured electrode. a Schematic illustration of the fabrication of a coaxial-shaped CNT/MnO2 electrode with a Ni fiber as the current corrector. b Line-scan EDS data on a cross-section image of a typical CNT/MnO2 hybrid nanostructured electrode (9-layered MnO2 and 8-layered CNTs) showing C (carbon, red line), Mn (manganese, green line), and O (oxygen, blue line) atoms (scale bar, 2.5 μm). c EDS elemental mappings for C, Mn, and O, respectively (scale bar, 3 μm). d A cross-sectional SEM image (scale bar, 2 μm) obtained by polishing with a cross-section polisher. e Schematic illustration of the configuration of the CNT/MnO2 hybrid nanostructures. f A cross-sectional SEM image of fracture end area of the CNT/MnO2 hybrid nanostructures (scale bar, 250 nm)
As mentioned above, the thickness of the MnO2 domains deposited electrochemically depends on the CV scan rate. For example, by reducing the CV scan rate and increasing the deposition time, thick nanostructured MnO2 domains can be deposited. The electrochemical conditions used in this study are shown in Supplementary Figure 2. It is noteworthy that our CNT/MnO2-based electrodes showed electrochemical properties that were superior when compared to those based only on MnO2 (Supplementary Figure 2a-d), especially for the electrodes constructed using low CV scan rates (10, 5, 2 mV s−1). Furthermore, a huge difference in the specific capacitances was observed (Supplementary Figure 2e). For the CNT/MnO2 electrodes prepared at CV scan rates of 2 mV s−1, specific capacitances of 9902 μF cm−1 were achieved, whereas a CNT-free MnO2 electrode showed values of 1803 μF cm−1. SEM images (Supplementary Figure 3) revealed cracked structures for the CNT-free MnO2-based electrodes prepared at CV scan rates of ≤20 mV s−1 (Supplementary Figure 3a-c). The same situation was observed in previous studies31. For our CNT/MnO2 hybrid nanostructures, as can be seen from the SEM images (Supplementary Figure 3d-f), the MnO2 domains were heavily covered by interconnected CNT networks (Supplementary Figure 3g-i), and the transfer of electrons among all the MnO2 domains can rapidly occur through the CNT networks. The nanostructured MnO2 domains resemble tissue cells while the heavily interconnected CNT networks are analogs of capillaries. This unique hybrid architecture enabled both high electrochemical reactivity and robust mechanical properties. The thickest CNT/MnO2 hybrid nanostructure we studied was 149 μm thick, and it was obtained after 10 electrochemical cycles for depositing MnO2 at a CV scan rate of 2 mV s−1, and 9 cycles for the CNT deposition. The actual amount of MnO2 being loaded in the ten-layered CNT/MnO2 hybrid electrode was estimated to be 2.06 mg cm−1. The capacitance values for different cycles for depositing MnO2 and CNT networks at CV scan rates of 2, 5, 10, and 50 mV s−1 are shown in the Supplementary Figure 4a. The specific capacitance for all electrodes was proportional to the CNT/MnO2 thickness. The presence of CNT networks allowed MnO2 domains to have full contributions to the capacitance (Supplementary Figure 4b). As mentioned above, the ratio of MnO2 within the CNT/MnO2 hybrid nanostructures varies as a function of the CV scan rate, estimated to be 49.39 wt%, 66.13 wt%, 82.99 wt%, 90.71 wt%, 95.13 wt%, and 97.99 wt% for scan rates of 100, 50, 20, 10, 5, and 2 mV s−1, respectively. These values were calculated from EDS analysis for each CNT/MnO2 sample. Thus, for the 2 mV s-1 deposited shell, the weight and capacitance of active material are both dominated by MnO2, even though CNT plays important role for mechanical support and electron percolation.
Nanostructured MnO2 domains embedded within CNT/MnO2 hybrids produced via electrochemical deposition exhibit the δ-crystalline phase, confirmed by X-ray diffraction (XRD) patterns (Supplementary Figure 5). As redox-active materials, δ- and α-crystalline nanostructured MnO2 always resulted in the best capacitance values (γ, λ, and then β)32,33. The MnO2 domains involved in the CNT/MnO2 hybrid nanostructures were built up by aggregating MnO2 nanoparticles. The average grain size of MnO2 nanoparticles were in the range of 5–30 nm, and strongly dependent on the CV scan rate.
Mechanism of energy storage for the CNT/MnO2 hybrid nanostructures
Similar to MnO2-based pseudo-capacitive materials, our CNT/MnO2 hybrid nanostructures store energy via both redox interactions and ion intercalation/deintercalation. Equation (1) describes the chemical description for the energy storing mechanism34,35:
where E+ denotes the cations of electrolyte. Mn(IV) in MnO2 displays a high oxidation state, having a higher potential than that of E+ during charging the supercapacitor. In other words, Eq. (1) can be rearranged in to Eq. (2), where E+ remained largely as cations but being electrically balanced by electrons, e−:
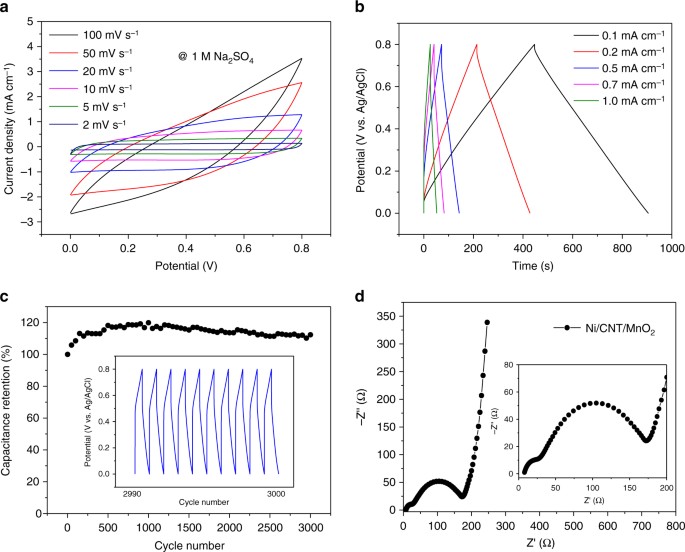
where [Mn(III)OO]–…E+ denotes an electrically induced intermediated state which stores electrons, i.e., energies via redox and ion-intercalation interactions. However, due to the very poor electrical conductivity of MnO2, the formation of [Mn(III)OO]–…E+, i.e., the redox and intercalation interactions, is restricted to a very thin MnO2 subsurface (ca. 420 nm34). Our CNT/MnO2 hybrid nanostructures, as can be seen from Fig. 1d, is novel and desirable since it is able to maximize the formation of [Mn(III)OO]–…E+. It is also noteworthy that the thickness of MnO2 domains can be precisely tuned at ca. 500 nm by controlling the CV scan rate and, moreover, electrons involved in [Mn(III)OO]–…E+ can be effectively delivered via the CNT networks. In other words, the CNT networks functioned as “electrical capillaries” which deliver electrons to nanostructured MnO2 domains. High capacitances can be achieved by increasing the overall thickness of MnO2 domains within the CNT/MnO2 hybrid nanostructures. Supplementary Figure 6 shows typical plots of the specific capacitance vs. thickness of the CNT/MnO2 hybrid nanostructures. The Ni/CNT/MnO2 electrode having a 149 μm thick CNT/MnO2 shell with 2.06 mg cm−1 MnO2 loading exhibited capacitances of 231.2 mF cm−1. The best reported data for the specific capacitance in MnO2 using CNT sheets was 60.6 mF cm−1, which is about one-fourth the value of the performance of our CNT/MnO2 multi-layered structure. Figure 2 shows CV curves (Fig. 2a), galvanostatic charge/discharge curves (Fig. 2b), cycling performance (Fig. 2c), and electrochemical impedance spectroscopy (EIS) spectra (Fig. 2d) for the coaxial Ni/CNT/MnO2 electrodes. All experiments were carried out using a three-electrode system in a 1.0 M Na2SO4 solution. The Ni/CNT/MnO2 hybrid nanostructured electrode used in Fig. 2 was electrochemically prepared using CV scan rates of 10 mV s−1, and the overall thickness of the CNT/MnO2 hybrid nanostructure was about 48 μm. Quasi-rectangular-shaped CV curves were observed when the CV scan rates ranged from 2 mV s−1 to 100 mV s−1 (Fig. 2a), as well as symmetrically shaped galvanostatic charge/discharge curves (Fig. 2b), thus indicating the presence of an ideal pseudo-capacitive material29,36. The specific capacitance vs. current densities for this Ni/CNT/MnO2 electrode was calculated based on the galvanostatic charge/discharge curves. It was found to be 51.3, 49.1, 44.1, 37.5, and 36.2 mF cm−1 for current densities of 0.1, 0.2, 0.5, 0.7, and 1.0 mA cm−1, respectively. The specific capacitance in weight of a 10-layered (CV rate for electrochemical deposition, 10 mV s−1) CNT/MnO2 hybrid electrode was calculated to be 236.5 F g−1 at a current density of 0.1 mA cm−1 by the mass of CNT/MnO2 hybrid nanostructuresd shell. Long charge/discharge cycling tests were performed at a current density of 2 mA cm−1, and the electrochemical stability of the CNT/MnO2 hybrid nanostructures remained nearly constant even after 3000 cycles (Fig. 2c). We also measured the diameter of the electrode after certain cycles of charging and discharging processes; changes were not observed, indicating the excellent durability of the CNT/MnO2 hybrid electrode. The CNT/MnO2 hybrid electrode remained stable while the working potential for the supercapacitor ranged from 0 V to 0.8 V (vs. Ag/AgCl). In the EIS measurements, the Nyquist plots (Fig. 2d) showed a vertical line in a low-frequency region and the low equivalent series resistance was calculated to be 6.8 Ω cm−1.
Electrochemical performance of a Ni/CNT/MnO2 electrode. a CV curves measured at different scan rates in 1.0 M Na2SO4. b Galvanostatic charge/discharge curves at different current densities. c Cycling performance at 2 mA cm−1; inset: galvanostatic charge/discharge curves from the 2990th to 3000th cycle. d EIS spectrum of the same Ni/CNT/MnO2 electrode; inset: the high-frequency region (Ni/CNT/MnO2 electrode, 9-layerd MnO2 and 9-layered CNTs obtained using CV scan rate at 10 mV s−1, thickness, 48 μm)
Symmetric supercapacitor devices
Two-electrode-type supercapacitors were assembled and the typical electrochemical responses are shown in Fig. 3. Cathode and anode consisted of a single piece of a Ni/CNT/MnO2 fiber having an identical shell thickness at around 48 μm and lengths of 1.15 cm; a 1.0 M Na2SO4 aqueous solution was used as the electrolyte. Interestingly, this device gives quasi-rectangular-shaped CV curves (Fig. 3a) and symmetrically shaped galvanostatic charge/discharge curves (Fig. 3b), indicating excellent capacitive performances. Cell linear capacitance remained at 76.3% (from 27.0 to 20.6 mF cm−1), even as the current density increased from 0.02 to 0.5 mA cm−1 (Fig. 3c). Energy density and power density of this two-electrode device were calculated to be 2.09 μWh cm−1 and 7.11 μW cm−1, respectively, at 0.02 mA cm−1, and 1.59 μWh cm−1 and 275.6 μW cm−1, for current densities of 0.5 mA cm−1 (Fig. 3d). Typical data on cell energy density and power density reported by other groups7,9,16, are also given in Fig. 3d for comparison. Our CNT/MnO2 hybrid nanostructures clearly show the best performance. Finally, two-electrode devices using Ni/CNT/MnO2 electrodes having the maximum CNT/MnO2 shell thickness (149 μm) resulted in energy densities of 10.97 μWh cm−1, which, to the best of our knowledge, are the best data on the MnO2-based FSCs reported hitherto. Energy density has been further enhanced by using bundled Ni/CNT/MnO2 fibers as electrodes (cathode and anode). In this context, a symmetrical supercapacitor with a triple-bundled Ni/CNT/MnO2 fiber (three strands of the 1.15-cm long Ni/CNT/MnO2 fibers were bundled in parallel and were then used as both cathode and anode) as electrodes was assembled. Surprisingly, this was capable of powering a light-emitting diode (LED; Fig. 4a). Note here that three micro-supercapacitor devices connected in parallel were also capable of powering the LED (Supplementary Figure 7). From the CV (Fig. 4b) and galvanostatic charge/discharge curves (Fig. 4c), the capacitance was enhanced nearly twice and three times when the dual and the triple-bundled Ni/CNT/MnO2 electrodes were used. The measured resistance (Fig. 4d) was 62.5 Ω, 37.5 Ω, and 12.5 Ω, for the single, dual-bundled, and the triple-bundled electrodes, respectively. In other words, bundled Ni/CNT/MnO2 electrodes result in high energy densities and high power densities, and also lower the internal resistance values of the pseudo-capacitive materials.
Electrochemical performance of a symmetric fiber-shaped supercapacitor device. a CV curves of the symmetric supercapacitor device (cathode and anode: Ni/CNT/MnO2, 9-layered MnO2 and 9-layered CNTs, total thickness, 48 μm) measured at various scan rates in 1.0 M Na2SO4. b Galvanostatic charge/discharge curves and c device capacitance of the symmetric supercapacitor device at various current densities. d Energy density and power density of the same supercapacitor device compared with typical published data on the fiber-shaped supercapacitor devices
Assembly of bundled Ni/CNT/MnO2 electrodes for a supercapacitor device. a A digital photograph of a light-emitting diode (LED) powered by a triple-bundled two-electrode type of a symmetric supercapacitor device. b CV curves, c galvanostatic charge/discharge curves and d EIS spectra of a single, dual-, and triple-bundled Ni/CNT/MnO2 electrode (9-layered MnO2 and 9-layered CNTs, the total thickness is 48 μm)
Discussion
It is clear that our fiber supercapacitors are potentially capable of driving wearable electronic devices. We established a novel CNT/MnO2 hybrid nanostructured material that resembles tissue cells and capillaries. In our case, CNT networks entangle within nanostructured MnO2 domains, thus allowing electrons to be injected effectively via these conducting CNT networks. Pseudo-capacitive domains have been activated via Faradic and the intercalation/deintercalation interactions, and the capability for storing energy therefore has been enhanced. Moreover, the self-conducting properties of our CNT/MnO2 hybrid nanostructures enabled us to increase the absolute energy density via the maximized loading rate of MnO2. The bending stability of the electrode depends entirely on the properties of the current collectors. CNT/MnO2 hybrid electrodes with carbon fibers as current correctors showed superior bending stabilities; they were able to bend 360 degrees while the electrochemical performance remained unchanged. Other combinatorial nanostructures, such as CNT/TiO2, CNT/RuO2, CNT/PbO2, CNT/Ni(OH)2, and CNT/Co(OH)2, are also possible via the electrochemical-deposition/CNT network formation methodology. We believe that our results pave the way to the design and development of novel wearable energy storage devices.
Methods
Preparation of aqueous dispersions containing mono-dispersed CNTs
As-grown, entangled multi-walled CNT powders (NC7000TM), were purchased from Nanocyl S.A. (Belgium). They were further dispersed into aqueous suspensions via the following steps: (i) 40 g of the as-purchased CNT powders were pre-dispersed into 1000 ml deionized water using a ball mill system (Multi Lab DYNO-Mill, 0.6 mm zirconium beads), (ii) 10 g of sodium cholate (Wako Chemicals) was then introduced and the slurry was then ball milled for about 50 min, (iii) 5.0 g of Polyvinylpyrrolidone (PVP) (Wako Chemicals) and 2.0 g of hydroxypropyl cellulose (Wako Chemicals) were introduced and the slurry was further ball milled till the CNTs disperse individually. Degrees of dispersion (D90 < 60 nm) were confirmed by a series of size distribution analysis using a dynamic light scattering analyzer (HORIBA Dynamic Light Scattering Particle Size Analyzer LB-550). A typical SEM image showing CNTs being dispersed individually is given in Supplementary Figure 8. Cholate functioned as the dispersing agent while PVP and hydroxypropyl cellulose functioned as the stabilizers.
Preparation of the Ni/CNT/MnO2 electrodes
Nanostructured MnO2 domains were anodically electrodeposited onto CNT networks via a cyclic voltammetry. A Ni mono-fiber (diameter, 200 μm) was used as the working electrode, a platinum foil as the counter electrode, and an Ag/AgCl electrode as the reference electrode. The Ni fiber was immersed into an aqueous solution containing 0.1 M Mn(Ac)2 and 0.1 M Na2SO4. Cyclic voltammograms were recorded between 0.4 and 1.4 V (vs. Ag/AgCl) at a certain scan rate (from 2 to 100 mV s−1) for 1 cycle. After electrodepositing the nanostructured MnO2 domains, the Ni/MnO2 electrode was rinsed in deionized water and then dried at room temperature. The Ni/MnO2 electrode was dipped into the mono-dispersed CNT suspension for 10 s and then rapidly removed. Subsequently, the Ni/CNT/MnO2 electrode was dried using a heat gun. After drying, the Ni/CNT/MnO2 electrode was subjected to surfactant/stabilizer removal by washing with abundant ethanol and deionized water, and dried at room temperature. The two steps were repeated several times until desirable Ni/CNT/MnO2 hybrid nanostructured electrodes were obtained. Four 10-layered CNT/MnO2 hybrid electrodes (CV scan rate for electrochemical deposition, 10 mV s−1) were prepared under identical experimental conditions and the energy density of each electrode was measured. The standard deviation (n = 4) was found to be 5.7%, indicating the CNT/MnO2 hybrid electrodes are reproducible.
Assembly of the symmetric supercapacitors
Two Ni/CNT/MnO2 hybrid nanostructured electrodes were placed parallel and ca. 500 μm apart, then fixed with tape and inserted into a flexible plastic tube filled with a 1.0 M Na2SO4 aqueous solution. The tube was finally sealed with epoxy resin to prepare the symmetric devices. One end of each electrode was connected to a 180 μm diameter Cu wire using silver paste for electrochemical performance measurements.
Characterization
The materials were characterized by XRD pattern (R-AXIS RAPID-SH, Rigaku) using a Mo radiation (Kα, λ = 0.071069 nm), field emission SEM (Hitachi SU-8020) equipped with an energy-dispersive X-ray spectroscopy (HORIBA X-Max) and SEM (JEOL JSM-6390, Japan). The cross-sectioned electrodes were prepared using a cross-section polisher (CP, JEOL SM-09010). All electrochemical measurements including CV curves, galvanostatic charge/discharge curves, EIS (that was obtained with a sinusoidal potential excitation of 5 mV in the frequency range from 100 kHz to 0.01 Hz), and cycling performance measurements were carried out using an electrochemical workstation (CHI 760E, CH Instruments). The electrochemical performance of the electrode materials was measured in a three-electrode system with 1.0 M Na2SO4 electrolyte solution. Electrode materials, a platinum foil, and an Ag/AgCl electrodes were used as working electrodes, counter electrodes, and reference electrodes, respectively. The electrochemical performance of the symmetric supercapacitors was evaluated in a two-electrode system. Two symmetry fiber electrodes of the same length (1.15 cm) and from the same synthesis batch were immersed into a beaker containing 1.0 M Na2SO4 solution, with a distance of about 10 mm.
Calculation of the electrochemical performances
The specific linear capacitance of the single electrode-based pseudo-capacitive material (C l ) in a three-electrode cell was calculated from galvanostatic charge/discharge curves, according to the following equation7:
where I denotes the constant discharge current; Δt denotes the time for a full discharge; l is the length of pseudo-capacitive electrode; and ΔV is the voltage drop on discharge (excluding the Vdrop).
The capacitance of the supercapacitor device (Ccell) in a two-electrode cell was calculated from their galvanostatic charge/discharge curves at different current densities based on the following equation14:
where i is the discharging current and dV/dt is the slope of the discharge curve.
The device linear capacitances of the FSCs (Ccell,l) was calculated according to the following equation:
where l refers to the device length of the FSCs.
The linear energy density of the FSCs (Ecell,l) was obtained from the following equation:
where ΔE is the operating voltage window in volts.
The linear power density of the FSCs (Pcell,l) was calculated from the galvanostatic curves at different charge/discharge current densities by using the following equation:
where tdischarge is the discharge time.
The overall capacitance of the CNT/MnO2 hybrid electrode increased proportionally as the length of the electrode increased. Due to the limitation of the surface area of the counter electrode, the maximum length of the CNT/MnO2 hybrid electrode prepared in this study was restricted to 5.2 cm.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Yu, D. et al. Emergence of fiber supercapacitors. Chem. Soc. Rev. 44, 647–662 (2015).
Jost, K., Dion, G. & Gogotsi, Y. Textile energy storage in perspective. J. Mater. Chem. A 2, 10776–10787 (2014).
Cai, X., Peng, M., Yu, X., Fu, Y. & Zou, D. Flexible planar/fiber-architectured supercapacitors for wearable energy storage. J. Mater. Chem. C 2, 1184–1200 (2014).
Lee, J. A. et al. Ultrafast charge and discharge biscrolled yarn supercapacitors for textiles and microdevices. Nat. Commun. 4, 1970 (2013).
Choi, C. et al. Flexible supercapacitor made of carbon nanotube yarn with internal pores. Adv. Mater. 26, 2059–2065 (2014).
Xiao, X. et al. Fiber-based all-solid-state flexible supercapacitors for self-powered systems. ACS Nano 6, 9200–9206 (2012).
Le, V. T. et al. Coaxial fiber supercapacitor using all-carbon material electrodes. ACS Nano 7, 5940–5947 (2013).
Yu, N. et al. High-performance fiber-shaped all-solid-state asymmetric supercapacitors based on ultrathin MnO2 nanosheet/carbon fiber cathodes for wearable electronics. Adv. Energy Mater. 6, 1501458 (2016).
Xu, P. et al. Stretchable wire-shaped asymmetric supercapacitors based on pristine and MnO2 coated carbon nanotube fibers. ACS Nano 9, 6088–6096 (2015).
Kou, L. et al. Coaxial wet-spun yarn supercapacitors for high-energy density and safe wearable electronics. Nat. Commun. 5, 3754 (2014).
Choi, C. et al. Improvement of system capacitance via weavable superelastic biscrolled yarn supercapacitors. Nat. Commun. 7, 13811 (2016).
Jiang, W. et al. Space-confined assembly of all-carbon hybrid fibers for capacitive energy storage: realizing a built-to-order concept for micro-supercapacitors. Energy Environ. Sci. 9, 611–622 (2016).
Chen, T., Hao, R., Peng, H. & Dai, L. High-performance, stretchable, wire-shaped supercapacitors. Angew. Chem. Int. Ed. 54, 618–622 (2015).
Yu, D. et al. Scalable synthesis of hierarchically structured carbon nanotube-graphene fibres for capacitive energy storage. Nat. Nanotechnol. 9, 555–562 (2014).
Yang, Z., Deng, J., Chen, X., Ren, J. & Peng, H. A highly stretchable, fiber-shaped supercapacitor. Angew. Chem. Int. Ed. 52, 13453–13457 (2013).
Ren, J., Bai, W., Guan, G., Zhang, Y. & Peng, H. Flexible and weaveable capacitor wire based on a carbon nanocomposite fiber. Adv. Mater. 25, 5965–5970 (2013).
Fu, Y. et al. Fiber supercapacitors utilizing pen ink for flexible/wearable energy storage. Adv. Mater. 24, 5713–5718 (2012).
Li, M., Zu, M., Yu, J., Cheng, H. & Li, Q. Stretchable fiber supercapacitors with high volumetric performance based on buckled MnO2/oxidized carbon nanotube fiber electrodes. Small 13, 1602994 (2017).
Choi, C. et al. Elastomeric and dynamic MnO2/CNT core-shell structure coiled yarn supercapacitor. Adv. Energy Mater. 6, 1502119 (2016).
Yu, Z. & Thomas, J. Energy storing electrical cables: integrating energy storage and electrical conduction. Adv. Mater. 26, 4279–4285 (2014).
Yu, D. et al. Controlled functionalization of carbonaceous fibers for asymmetric solid-state micro-supercapacitors with high volumetric energy density. Adv. Mater. 26, 6790–6797 (2014).
Choi, C. et al. Microscopically buckled and macroscopically coiled fibers for ultra-stretchable supercapacitors. Adv. Energy Mater. 7, 1602021 (2017).
Ren, J. et al. Twisting carbon nanotube fibers for both wire-shaped micro-supercapacitor and micro-battery. Adv. Mater. 25, 1155–1159 (2013).
Meng, Y. et al. All-graphene core-sheath microfibers for all-solid-state, stretchable fibriform supercapacitors and wearable electronic textiles. Adv. Mater. 25, 2326–2331 (2013).
Chen, X. et al. Novel electric double-layer capacitor with a coaxial fiber structure. Adv. Mater. 25, 6436–6441 (2013).
Liu, L., Yu, Y., Yan, C., Li, K. & Zheng, Z. Wearable energy-dense and power-dense supercapacitor yarns enabled by scalable graphene-metallic textile composite electrodes. Nat. Commun. 6, 7260 (2015).
Wang, G., Zhang, L. & Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 41, 797–828 (2012).
Lima, M. et al. Biscrolling nanotube sheets and functional guests into yarns. Science 331, 51–55 (2011).
Chou, S. L., Wang, J. Z., Chew, S. Y., Liu, H. K. & Dou, S. X. Electrodeposition of MnO2 nanowires on carbon nanotube paper as free-standing, flexible electrode for supercapacitors. Electrochem. Commun. 10, 1724–1727 (2008).
Zheng, H. et al. Layer-by-layer assembly and electrochemical properties of sandwiched film of manganese oxide nanosheet and carbon nanotube. Carbon 47, 1534–1542 (2009).
Chen, Y. C. et al. Highly flexible supercapacitors with manganese oxide nanosheet/carbon cloth electrode. Electrochim. Acta 56, 7124–7130 (2011).
Brousse, T. et al. Crystalline MnO2 as possible alternative to amorphous compounds in electrochemical supercapacitors. J. Electrochem. Soc. 153, A2171–A2180 (2006).
Devaraj, S. & Munichandraiah, N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J. Phys. Chem. C 112, 4406–4417 (2008).
Toupin, M., Brousse, T. & Bélanger, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 16, 3184–3190 (2004).
Ghodbane, O., Ataherian, F., Wu, N. L. & Favier, F. In situ crystallographic investigations of charge storage mechanisms in MnO2-based electrochemical capacitors. J. Power Sources 206, 454–462 (2012).
Su, Z. et al. Scalable fabrication of MnO2 nanostructure deposited on free-standing Ni nanocone arrays for ultrathin, flexible, high-performance micro-supercapacitor. Energy Environ. Sci. 7, 2652–2659 (2014).
Acknowledgements
This research is supported in part by grants from the Project of Saitama Prefectural Industry-Accademia Collaborative Development Project Subsidy. J.L. acknowledges support by NSF ECCS-1610806. This research project started in 2015 as a collaborative research project with the laboratory of Mildred S. Dresselhaus (Institute Professor of Massachusetts Institute of Technology). Mildred S. Dresselhaus supervised this research project until she passed away in February 2017.
Author information
Authors and Affiliations
Contributions
W.G. and B.F. conceived the idea and designed the experiments. W.G. carried out most of the experimental works. Z.W. and H.O. carried out SEM and XRD. I.S., L.S., X.Z., M.L., C.W., J.L., J.O.-M., M.T., and M.E. discussed the interpretation of results and co-wrote the paper. All authors discussed the results commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gong, W., Fugetsu, B., Wang, Z. et al. Carbon nanotubes and manganese oxide hybrid nanostructures as high performance fiber supercapacitors. Commun Chem 1, 16 (2018). https://doi.org/10.1038/s42004-018-0017-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-018-0017-z
This article is cited by
-
Fabrication of Polypyrrole/Reduced Graphene Oxide/Silk as a High-Performance Electrode for Fiber-Shaped Flexible Supercapacitor
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
Micron/nanoporous architecture sculptured on Ni wire via direct-flame treatment with tunable porosity for fiber-shaped supercapacitors
Journal of Materials Science: Materials in Electronics (2023)
-
RETRACTED ARTICLE: Polydopamine-modified MWCNT/graphene oxide hybrid 3D carbon nano-structure for flexible symmetric supercapacitor electrodes
Applied Nanoscience (2023)
-
Synthesis of MnO2/PPy nanocomposites by novel emulsifying device and its electrochemical performances
Journal of Sol-Gel Science and Technology (2020)
-
Manganese oxide synthesized from spent Zn-C battery for supercapacitor electrode application
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.