Abstract
The ability to stack multiple genes in plants is of great importance in the development of crops with desirable traits but can be challenging due to limited selectable marker options. Here we establish split selectable marker systems using protein splicing elements called “inteins” for Agrobacterium-mediated co-transformation in plants. First, we show that such a split selectable marker system can be used effectively in plants to reconstitute a visible marker, RUBY, from two non-functional fragments through tobacco leaf infiltration. Next, to determine the general applicability of our split selectable marker systems, we demonstrate the utility of these systems in the model plants Arabidopsis and poplar by successfully stacking two reporters eYGFPuv and RUBY, using split Kanamycin or Hygromycin resistance markers. In conclusion, this method enables robust plant co-transformation, providing a valuable tool for the simultaneous insertion of multiple genes into both herbaceous and woody plants efficiently.
Similar content being viewed by others
Introduction
Metabolic engineering in plants relies on the introduction of complete or partial synthetic pathways into the target plant to create novel plant traits and produce value-added metabolites and therapeutic proteins1,2. A complete synthetic pathway is typically encoded by multiple genes that involve multiple genetic parts and gene circuits. Multigene engineering, therefore, is becoming more and more important for plant synthetic biology research. Also, a lot of complex plant traits (e.g., yield) are controlled by multiple genes, and as such, genetic improvement of such polygenic traits requires multi-gene stacking. Agrobacterium-mediated transformation is, to date, the most widely used method for plant genetic engineering due to its relatively high efficiency3. Although some progress in Agrobacterium-mediated transformation of large DNA fragments required for multigene engineering in plants has been achieved, it has been reported that large genomic DNA fragments are not stable in Agrobacterium, and T-DNA can be truncated at the left and/or right ends before being inserted into the plant genome4,5,6. Thus, the effective transformation of tens of genes into a plant genome and consequent optimal control of gene expression remain unattainable in plant engineering. Here, we developed a split-intein-based gene-stacking method through split-selectable-marker-enabled co-transformation in Arabidopsis thaliana and poplar (Populus).
An intein is an intervening protein domain that excises itself post-translationally from the host protein leading to the ligation of flanking N- and C-terminal residues (termed as exteins), which share a common intein, to form a new protein (Fig. 1a)7. Split inteins to date have enabled the development of numerous tools for both synthetic and biological applications by providing a rapid and bioorthogonal means to link two polypeptides7,8. As such, we previously showed that split intein, derived from NpuDnaE9, can be used to reduce the size of the CRISPR/Cas9 system by splitting Cas9 into multiple fragments, leading to effective base editing in plants10. In this study, we aim to develop split selectable markers using split inteins to enable single-selectable-marker-gene dependent co-transformation in plants.
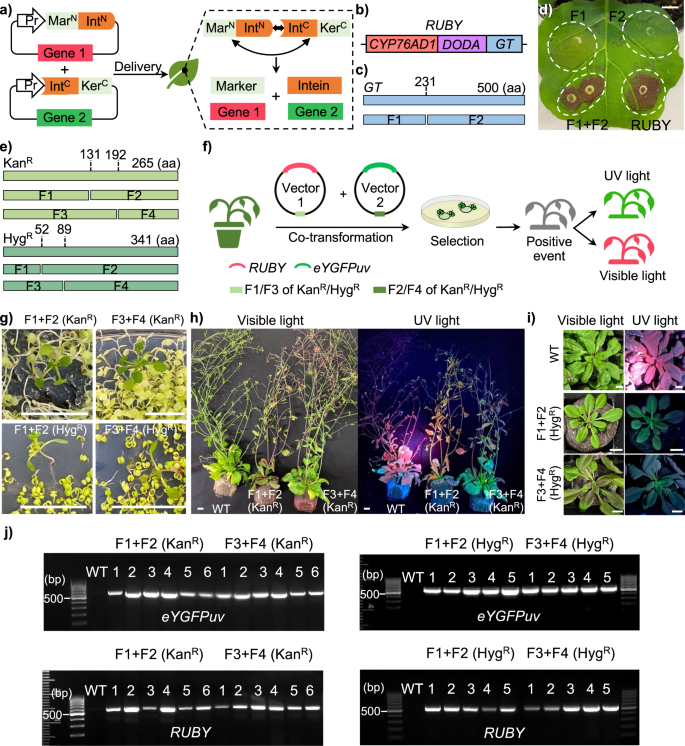
a Split selectable marker for co-selection of two separate transgenic vectors. b Illustration of RUBY reporter. c Identification of the potential split site of gene GT. d Transient expression of split-RUBY in N. benthamiana. Scale bar, 1 cm. e Identification of potential split sites of gene KanR and HygR. f Experiment design of the split–selectable markers mediated co-transformation in plants. g Selection of T1 seedling on MS medium containing Kanamycin or Hygromycin. Scale bar, 1 cm. h Phenotyping of Kanamycin resistant T1 transformants (6 weeks). Scale bar, 1 cm. i Phenotyping of Hygromycin resistant T1 transformants (four weeks). Scale bar, 1 cm. j Genotyping of T1 transformants using primers of eYGFPuv and RUBY, respectively.
Results
Intein-mediated split RUBY reporter in tobacco leaf infiltration
Initially, we selected eYGFPuv11 and RUBY12 as the reporter genes that can be easily visualized by naked eyes with and without UV light, respectively, to establish a functional split system. In fact, the RUBY reporter is encoded by three genes CYP76AD1, DODA, and glucosyltransferase (GT) (Fig. 1b). In general, a catalytic Cys residue at position +1 of the C-terminal extein is mandatory to maintain substantial splicing activities7. A potential split site, L231:C232, was thus identified for splitting the gene GT into two fragments, termed GTf1 and GTf2 (Fig. 1c). We split the RUBY reporter into two parts by creating plasmid pAXY0006 containing CYP76AD1, DODA and GTf1-NpuDnaE(N), and plasmid pAXY0007 containing NpuDnaE(C)-GTf2 (Supplementary Fig. 1). Note that the Arabidopsis codon-optimized NpuDnaE intein was created to improve gene expression and translational efficiency in plants. We then tested split-RUBY using Agrobacterium-mediated leaf infiltration in Nicotiana benthamiana. The strong red pigment was observed by naked eyes both in the positive control (RUBY) and the leaf area co-infiltrated with pAXY0006 and pAXY0007 plasmids, whereas no red pigment was detected in the leaf area infiltrated with pAXY0006 or pAXY0007 plasmids alone (negative controls) (Fig. 1d). Taken together, our results indicate that the functional RUBY reporter was restored by split inteins, which is consistent with the split-eYGFPuv reported previously10.
The intein-mediated split selectable marker in Arabidopsis
Because Kanamycin resistance (KanR; nptII) and Hygromycin resistance (HygR; hpt) are widely used as the selectable markers in plant transformation, we next tested NpuDnaE intein9 for splitting the nptII gene encoding neomycin phosphotransferase II and the hpt gene encoding Hygromycin phosphotransferase, which confers KanR and HygR, respectively. Following the rule of obligatory cysteine residue on the C-extein7, we identified two split sites (T131:C132 and A192:C193) for nptII and two split sites (S52:C53 and Y89:C90) for hpt (Fig. 1e).
The coding sequence of nptII or hpt was split into an N-terminal fragment (MarN, F1/F3) and a C-terminal fragment (KerC, F2/F4), which were then cloned upstream of an N-terminal fragment of the NpuDnaE intein (IntN) and downstream of a C-terminal fragment of the NpuDnaE intein (IntC), respectively, into two vectors (Fig. 1a, e). Each vector also carries one of the two reporters (eYGFPuv and RUBY), which allows for easy assessment of co-transformation (Supplementary Fig. 2). Thus, we expect to see both green fluorescences under UV light and red pigment under visible light in Kanamycin-resistant or Hygromycin-resistant transgenic plants after co-transformation of these two vectors (Fig. 1f).
After co-transformation via a floral dip in Arabidopsis, multiple transgenic seedlings with typical Kanamycin-resistant or Hygromycin-resistant phenotype were successfully identified on the selection media, indicating that the two inactive fragments of each selectable marker gene (nptII or hpt) were effectively reconstituted post-translationally (Fig. 1g). Subsequently, the green fluorescence and red pigment were observed at different stages of Kanamycin-resistant T1 plants (Fig. 1h) and Hygromycin-resistant T1 plants (Fig. 1i) under UV light and visible light, respectively, suggesting that both eYGFPuv and RUBY vectors were also transformed into the same plant simultaneously through split-KanR- or split-HygR-mediated co-transformation. These observations were further confirmed by polymerase chain reaction (PCR)-based genotyping, where both eYGFPuv and RUBY genes were detected in all Kanamycin-resistant or Hygromycin-resistant plants (Fig. 1j and Supplementary Fig. 3). Next, we evaluated the phenotype of T2 generations of above transgenic plants, with the expectation that the Kanamycin-resistance or Hygromycin-resistance phenotype will be observed in T2 seedlings, along with green fluorescence under UV light and red pigment under visible light, as seen in Fig. 2a. Due to segregation of T-DNA inserts in the offspring, not all T2 plants exhibit the phenotype of antibiotic resistance and expression of both eYGFPuv and RUBY, but the phenotype of eYGFPuv and RUBY was observed in most resistant T2 plants though the expression level varies among plants (Fig. 2a). The phenotypes of eYGFPuv and RUBY were continuously detected in the mature plants of Kanamycin-resistant lines and Hygromycin-resistant lines (Fig. 2b and Supplementary Fig. 4). These findings were also supported by PCR-based genotyping, which revealed the presence of the eYGFPuv and RUBY genes in every plant that was either kanamycin- or hygromycin-resistant (Supplementary Fig. 5). Taken together, the traits of Kanamycin-resistant, Hygromycin-resistant, eYGFPuv and RUBY are all heritable in Arabidopsis across generations, demonstrating the split–KanR- and split–HygR-mediated co-transformation are effective and robust methods for stable gene-stacking in plants.
The intein-mediated split selectable marker in poplar
We further examined the efficacy of split–HygR system in poplar using vector pairs F3 and F4. After tissue-culture-based co-transformation in Poplar ‘717’ (Populus tremula x alba clone INRA ‘717-1B4’), we observed more than 20 transgenic shoots that showed bright green fluorescence under UV light. We randomly selected 15 eYGFPuv-expressing shoots and cultured them on a root induction medium supplied with Hygromycin (Fig. 3a), where 80% of induced shoots were rooted successfully on the selection medium, suggesting that functional Hygromycin phosphotransferase was generated post-translationally. We observed consistent green fluorescence in all rooted plants over time though some plants showed weaker and nonuniform eYGFPuv signals (Fig. 3a). Interestingly, typical RUBY phenotype was not observed in the rooted plants though red pigmentation was observed in the stem of some plants. Based on the RUBY expression in transgenic poplar plants generated previously13, red pigment is typical to be visible in different organs, including leaf, stem, and root. However, the red pigmentation in the stem of some plants can also be caused by stress, which is not rare during plant transformation. Intriguingly, we detected both eYGFPuv and RUBY genes via PCR genotyping in all rooted plants (Fig. 3b, c and Supplementary Fig. 6). Overall, these results suggest that the split–HygR system can also work effectively in tissue-culture-based transformation in woody plants.
a Root induction in root induction medium supplied with Hygromycin and phenotyping of transgenic events with/without UV light. Scale bar, 1 cm. b Genotyping of transgenic poplar events using primers of eYGFPuv and RUBY, respectively. c The analysis and alignment of genotyping and phenotyping of transgenic plants. The blue block represents positive events, while the gray block represents negative events.
Western blot analysis of protein trans-splicing of Hygromycin markertrons
To directly observe protein splicing and to confirm these inteins are indeed orthogonal, we conducted western blot analysis of protein trans-splicing between N-HygR N-terminally tagged with 3xFLAG-epitope and C-HygR C-terminally tagged with 3xHA-epitope (Fig. 4 and Supplementary Figure 7). As expected, full-length HygR (lanes 6 and 7) was observed in the co-transformation of cognate HygR fragments with matching N- and C-inteins while transformation with fragment F1/F2/F3/F4 only (lanes 2–5) did not yield full-length HygR (Fig. 4b and Supplementary Fig. 8).
a The N-terminal fragments of HygR (F1 and F3) are N-terminally tagged with 3xFLAG epitope while the C-terminal fragments of HygR (F2 and F4) are C-terminally tagged with 3xHA epitope. b Western blot was performed with the proteins extracted from human kidney cells, which were either transfected with the plasmids containing one of the fragments F1–F4, respectively, or co-transfected with F1 + F2 containing plasmids and F3 + F4 containing plasmids. Red, green, and yellow bands indicate FLAG, HA, and merged bands, respectively. Actin serves as the equal-loading control. Note: Fig. 4b shows cropped, contrast-adjusted image. Please see the original images of Fig. 4b in Supplementary Fig. 8.
Discussion
Current plant co-transformation approaches rely on at least two selectable gene markers. For this protocol, the concentrations of combined antibiotics need to be tested and adjusted carefully to achieve optimal transgenic selection effect. There is also a difference in selection efficacy between different selectable markers, such that, HygR works better (lower rate of false positives) than KanR in the genetic transformation of some poplar genotypes14,15. In this study, for the first time in plants, we demonstrate that the systems of split–KanR and split–HygR are effective for both in planta and plant tissue culture co-transformation in herbaceous and woody plants.
By dividing the larger cargoes across two T-DNAs, such systems enable the effective co-transformation of two separate binary vectors into a plant by Agrobacterium-mediated transformation. One constraint is that the insertion sites of the two T-DNAs are not controlled. Thus the two T-DNAs will exhibit Mendelian segregation, as observed in Fig. 2a. In fact, most frequently, the offspring of crossings between different parents are used to study the inheritance of mutant traits in A. thaliana16. For example, by mating the two single knockout mutants, constitutive double knockout Arabidopsis mutants lacking both DPE2 and PHS1 were produced17. Yuan et al. generated a pp2ab’αβ double mutant by crossing two homozygous single-insertion mutants, pp2ab’α and pp2ab’β18. To develop homozygous drm1drm2 double mutant plants, Cao and Jacobsen crossed two isolated singe mutants drm1 and drm2, in order to examine the function of the DRM genes19. In general, a heterozygous double mutant will be generated in the F1 generation, which is equivalent to the T1 generation of this study, and a homozygous double mutant will be achieved in the F2 generation, which is equivalent to the T2 generation of this study. Therefore, a homozygous double mutant created by split–KanR or split–HygR system is expected to be achieved in T2 generation for plant species with sexual reproduction, e.g., Arabidopsis, rice, and tomato. In contrast, for plants relying on vegetative propagation, e.g., poplar and citrus, the phenotype of double mutant will be inherited consistently without phenotype segregation.
To easily identify transgenic events in plant transformation, we chose RUBY and eYGFPuv as the selectable reporters which are visible to the naked eye under white and UV light, respectively, without a need for cost- and labor-intensive characterization11,12,13. Indeed, the green fluorescence of plants expressing eYGFPuv can be observed consistently both in Arabidopsis and poplar. The red pigment of plants expressing RUBY, in contrast, was less consistent, particularly in poplar, where no typical RUBY phenotype was found in this study. To address this issue, a more reliable reporter such as GUS or LUC tends to be a better option to replace the RUBY reporter.
The advantages of these co-transformation methods can reduce valuable time spent on constructing complex or long T-DNA molecules in binary vectors and sequential transformations, thus improving the capabilities for pathway engineering and genetic improvement of polygenic traits. In addition, the current common practice of expressing multiple genes involves the repeated use of the same or similar promoters due to the limited number of available promoters20. Here, repetitive sequences within a plasmid can undergo intramolecular DNA recombination21. This scenario is avoided with the use of the split selectable marker system described here. The choice of delivering multiple gene expression cassettes containing multiple identical sequences with two transformation vectors should allow a drastic reduction in the frequency of plasmid DNA recombination. Finally, this technology potentially doubles the capacity of existing transformation systems for multi-gene engineering in plants.
Methods
Plant materials
Arabidopsis (A. thaliana) ecotype Columbia-0 (Col-0) and tobacco (Nicotiana benthamiana) were grown in controlled-climate chambers under fluorescent cold white light (100–150 µmol m−2 s−1), 16-h light/8-h dark photoperiod, 20–22 °C, and 60% humidity. In vitro-grown poplar ‘717’ (Populus tremula × P. alba clone INRA 717-1B4) plantlets were placed in a growth room with a photoperiod of 16-h light/8-h dark at 22 °C.
Vector construction
To split RUBY, we first created a RUBY-minus vector lacking the gene GT by assembling PCR product 1 containing CYP76AD1 and DODA and PCR product 2 containing Arabidopsis HSP18.2 terminator into a pGFPGUSplus vector22 via NEBuilder HiFi DNA Assembly (New England BioLabs). The pAXY0006 vector of split-RUBY was generated by assembling PCR products containing f1 fragment of gene GT (named GTf1) and NpuDnaE(N) into RUBY-minus vector via NEBuilder HiFi DNA Assembly. The pAXY0007 vector of split-RUBY was generated by assembling PCR products containing an f2 fragment of gene GT (named GTf2) and NpuDnaE(C) into pGFPGUSplus vector via NEBuilder HiFi DNA Assembly. To split KanR (i.e., nptII) and HygR (i.e., hpt), gBlocks Gene Fragments containing either 5′-KanR/HygR and N-terminal of NpuDnaE or C-terminal of NpuDnaE and 3′-KanR/HygR were synthesized from Integrated DNA Technologies IDT. The pAXY0008/00010/00012/00014 vectors of split-KanR/HygR were generated by assembling PCR products containing F1/F3 fragments of KanR/HygR and NpuDnaE(N) into pGFPGUSplus vector via NEBuilder HiFi DNA Assembly. The pAXY0009/00011/00013/00015 vectors of split-KanR/HygR were generated by assembling PCR products containing F2/F4 fragment of KanR/HygR and NpuDnaE(C) into pGFPGUSplus vector via NEBuilder HiFi DNA Assembly. The coding sequences of inteins were codon optimized for Arabidopsis via the online codon optimization tool (ExpOptimizer) provided by NovoPro Bioscience (Shanghai, China). All vectors were verified by Sanger sequencing. Information for all primers, gBlocks, and plasmids used in this study is provided in Supplementary Data 1 and Supplementary Table 1.
Arabidopsis stable transformation
The A. tumefaciens strain ‘GV3101’ was used for the transformation of Arabidopsis wild type ‘Col-0’ via the floral dip method as described previously23. For co-transformation, two Agrobacterium strains containing corresponding vectors (Supplementary Fig. 2), respectively, were cultured separately overnight in 100 mL LB liquid medium supplied with 50 mg/L Kanamycin and 50 mg/L Rifampicin. Two LB cultures were spun down at 4000–5000 rpm for 20 min and resuspended in 30 mL new LB liquid medium without antibiotics and mixed equally. Mixed LB culture was added into 120 mL dip solution containing 5% sucrose and 0.03% Silwet-L77. In general, 8–12 plants were used for each co-transformation.
Poplar stable transformation
The A. tumefaciens strain ‘EHA105’ was used for the co-transformation of the poplar ‘717’ following a published method24. 50 mL LB culture for each Agrobacterium strain was prepared and spun down as described above. Two Agrobacterium pellets were resuspended equally in an MS induction medium containing 20 μM acetosyringone at an OD600 nm of 0.5–0.8 for each strain. Excised leaf disks from young leaves (~150) were soaked in Agrobacterium solution for 1 h, followed by multiple steps, including co-culture, washing, callus induction, shoot induction, shoot elongation, and root induction.
Tobacco leaf infiltration
Infiltration of tobacco leaves was performed following a published method24. For co-infiltration, a 5 mL overnight culture of two Agrobacterium strains was spun down and resuspended equally in a resuspension solution containing 10 mM MgCl2, 10 mM MES-K (pH 5.6), and 100 μM acetosyringone at an OD600 nm of 0.5 for each strain.
Genotyping
To genotype the resistant lines, leaves, approximately 0.5–1.0 cm, were collected from Arabidopsis and poplar ‘717’ and ground to a powder. Genomic DNA was isolated by a modified sodium dodecyl sulfate (SDS)-based DNA extraction method25. Forward primer 5′-CACGGCAACCTCAACG-3′ and reverse primer 5′-CTCGACACGTCTGTGGG-3′ were used for genotyping PCR of eYGFPuv. Forward primer 5′- CAGAGCTTGCGAGAAAGG-3′ and reverse primer 5′- GGCGGAGGTGAACTTGTAG-3′ were used for genotyping PCR of RUBY.
Phenotyping
The fluorescence signals of eYGFPuv were visualized under a 365 nm wavelength UV light and imaged using an iPhone 11 as described by Yuan et al.11. The red pigment due to RUBY expression is visible by naked eyes without requiring any equipment12, and images were also taken using an iPhone 11.
Protein extraction and Western blot
HEK 293 T cells were obtained from ATCC and maintained in a humidified atmosphere at 5% CO2 in Dulbecco’s Modified Eagle’s (DMEM) complete medium (Corning) supplemented with 10% fetal bovine serum (FBS; Seradigm) in 37 °C. Plasmid transfections were done with TransIT-LT1 (Mirus Bio) per the manufacturer’s instructions. Briefly, cell extracts were generated on ice in EBC buffer, 50 mM Tris (pH 8.0), 120 mM NaCl, 0.5% NP40, 1 mM DTT, and protease and phosphatase inhibitors tablets (Thermo Fisher Scientific). Extracted proteins were quantified using the PierceTM BCA Protein assay kit (Thermo Fisher). Proteins were separated by SDS acrylamide gel electrophoresis and transferred to IMMOBILON-FL 26 PVDF membrane (Millipore) probed with the indicated antibodies and visualized either by chemiluminescence (according to the manufacturer’s instructions) or using a LiCor Odyssey infrared imaging system. Western blot was conducted as described previously10. Primary antibodies used for western blot are HA (Cat# 902302; 1:1000 dilution) from Biolegend and M2 FLAG (Cat# F1804; 1:1000 dilution) antibody from Sigma. Secondary antibodies used are IRDye 800CW Goat anti-Mouse IgG Secondary Antibody (LiCor) and IRDye 680RD Goat anti-Rabbit IgG Secondary Antibody (LiCor). The study used a primary antibody against β-actin (Cat# A1978 from Sigma) for internal protein control.
Statistics and reproducibility
The experiment of tobacco leaf infiltration was performed two times and with two replicates each time. The stable transformation of both Arabidopsis and poplar was conducted two times. For each Arabidopsis transformation, a minimum of eight individual plants were utilized. Similarly, at least 150 explants were employed for each poplar transformation. A minimum of five transgenic events were chosen for PCR and phenotype analysis in Arabidopsis, while fifteen transgenic events were selected for the same analysis in Poplar. The number of replicates was described in each figure and figure legend.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The plasmids will be available at Addgene. All other data are available from the corresponding author upon reasonable request.
References
Zhu, Q. et al. Plant synthetic metabolic engineering for enhancing crop nutritional quality. Plant Commun. 1, 100017 (2020).
Shih, P. M., Liang, Y. & Loqué, D. Biotechnology and synthetic biology approaches for metabolic engineering of bioenergy crops. Plant J. 87, 103–117 (2016).
Gelvin, S. B. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 67, 16–37 (2003).
Hamilton, C. M., Frary, A., Lewis, C. & Tanksley, S. D. Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc. Natl Acad. Sci. USA 93, 9975–9979 (1996).
Song, J., Bradeen, J. M., Naess, S. K., Helgeson, J. P. & Jiang, J. BIBAC and TAC clones containing potato genomic DNA fragments larger than 100 kb are not stable in Agrobacterium. Theor. Appl. Genet. 107, 958–964 (2003).
Miranda, A., Janssen, G., Hodges, L., Peralta, E. G. & Ream, W. Agrobacterium tumefaciens transfers extremely long T-DNAs by a unidirectional mechanism. J. Bacteriol. 174, 2288–2297 (1992).
Stevens, A. J. et al. A promiscuous split intein with expanded protein engineering applications. Proc. Natl Acad. Sci. USA 114, 8538–8543 (2017).
Shah, N. H. & Muir, T. W. Inteins: nature’s gift to protein chemists. Chem. Sci. 5, 446–461 (2014).
Jillette, N., Du, M., Zhu, J. J., Cardoz, P. & Cheng, A. W. Split selectable markers. Nat. Commun. 10, 4968 (2019).
Yuan, G. et al. An intein-mediated split–nCas9 system for base editing in plants. ACS Synth. Biol. 11, 2513–2517 (2022).
Yuan, G. et al. Expanding the application of a UV-visible reporter for transient gene expression and stable transformation in plants. Hortic. Res. 8, 234 (2021).
He, Y., Zhang, T., Sun, H., Zhan, H. & Zhao, Y. A reporter for noninvasively monitoring gene expression and plant transformation. Hortic. Res. 7, 152 (2020).
Yuan, G. et al. Reporter genes confer new-to-nature ornamental traits in plants. Hortic. Res. https://doi.org/10.1093/hr/uhac077 (2022).
Cseke, L. J., Cseke, S. B. & Podila, G. K. High efficiency poplar transformation. Plant Cell Rep. 26, 1529–1538 (2007).
Fan, D. et al. Efficient CRISPR/Cas9-mediated targeted mutagenesis in populus in the first generation. Sci. Rep. 5, 12217 (2015).
Weigel, D. & Glazebrook, J. Genetic analysis of Arabidopsis mutants. CSH Protoc. 2008, pdb.top35 (2008).
Malinova, I. et al. Double knockout mutants of Arabidopsis grown under normal conditions reveal that the plastidial phosphorylase isozyme participates in transitory starch metabolism. Plant Physiol. 164, 907–921 (2014).
Yuan, G. et al. PROTEIN PHOSHATASE 2A B’α and β maintain centromeric sister chromatid cohesion during meiosis in Arabidopsis. Plant Physiol. 178, 317–328 (2018).
Cao, X. & Jacobsen, S. E. Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr. Biol. 12, 1138–1144 (2002).
Tian, C., Zhang, Y., Li, J. & Wang, Y. Benchmarking intrinsic promoters and terminators for plant synthetic biology research. BioDesign Res. 2022, 9834989 (2022).
Bzymek, M. & Lovett, S. T. Instability of repetitive DNA sequences: the role of replication in multiple mechanisms. Proc. Natl Acad. Sci. USA 98, 8319–8325 (2001).
Vickers, C. E., Schenk, P. M., Li, D., Mullineaux, P. M. & Gresshoff, P. M. pGFPGUSPlus, a new binary vector for gene expression studies and optimising transformation systems in plants. Biotechnol. Lett. 29, 1793–1796 (2007).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Yuan, G., Tuskan, G. A. & Yang, X. Use of fluorescent protein reporters for assessing and detecting genome editing reagents and transgene expression in plants. In: Plant Genome Engineering: Methods and Protocols. 115–127 (New York, NY: Springer US, 2023).
Edwards, K., Johnstone, C. & Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 19, 1349 (1991).
Acknowledgements
The work was supported by the Center for Bioenergy Innovation, a U.S. Department of Energy (DOE) Bioenergy Research Center supported by the Biological and Environmental Research (BER) program. Oak Ridge National Laboratory is managed by UT-Battelle, LLC for the U.S. Department of Energy under Contract No. DE-AC05-00OR22725. This paper has been authored by UT-Battelle, LLC, under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this paper or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Author information
Authors and Affiliations
Contributions
G.Y. and X.Y conceived the research. G.Y. and H.L. conducted the plant transformation. K.D. performed Western blot. Y.L. and M.T.I. performed genotyping. G.Y. wrote the paper. M.M.H., W.M., G.A.T., and X.Y. revised the paper. X.Y. and G.A.T. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Pradeep Deo and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Leena Tripathi and David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, G., Lu, H., De, K. et al. Split selectable marker systems utilizing inteins facilitate gene stacking in plants. Commun Biol 6, 567 (2023). https://doi.org/10.1038/s42003-023-04950-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04950-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.