Abstract
Nitrogen isotopes are widely used to study the trophic position of animals in modern food webs; however, their application in the fossil record is severely limited by degradation of organic material during fossilization. In this study, we show that the nitrogen isotope composition of organic matter preserved in mammalian tooth enamel (δ15Nenamel) records diet and trophic position. The δ15Nenamel of modern African mammals shows a 3.7‰ increase between herbivores and carnivores as expected from trophic enrichment, and there is a strong positive correlation between δ15Nenamel and δ15Nbone-collagen values from the same individuals. Additionally, δ15Nenamel values of Late Pleistocene fossil teeth preserve diet and trophic level information, despite complete diagenetic loss of collagen in the same specimens. We demonstrate that δ15Nenamel represents a powerful geochemical proxy for diet that is applicable to fossils and can help delineate major dietary transitions in ancient vertebrate lineages.
Similar content being viewed by others
Introduction
Diet is a fundamental driver of evolution, and the development of geochemical proxies that can be used to reconstruct past food webs has been a central focus of paleontological research over the last several decades. While traditional stable isotope analyses of tooth enamel (e.g., carbon and oxygen) and trace element ratios (e.g., Sr/Ca and Ba/Ca) have significantly advanced our understanding of the dietary ecology of fossil organisms1,2, these proxies do not provide clear information about trophic level. In contrast, the nitrogen (N) isotope ratio of organic material (expressed as δ15N vs. AIR in ‰, where δ15N = [(15N/14Nsample/15N/14Nreference) − 1] × 1000) derived from bulk tissues or specific compounds (e.g., amino acids) is a widely used proxy for the dietary and trophic behavior of animals in terrestrial and marine food webs. More than 40 years of research shows that, in well-constrained systems, consumer tissues are typically enriched by 3 to 4‰ in 15N relative to their diet, and δ15N analyses have been applied across a broad range of ecosystems3,4,5,6,7,8,9,10.
Despite the potential of nitrogen isotopes for trophic reconstruction, their application in the paleontological record has been limited due to the poor preservation of N-containing organic matter. Nitrogen isotope studies of fossil vertebrates have largely been restricted to collagen extracted from relatively young (e.g., <100 ka), well-preserved bone or dentin samples11,12,13,14. While recent efforts to develop new trophic proxies have expanded the geochemical tools at our disposal (e.g., to include calcium and zinc isotopes), data documenting baseline variation and variability in the isotopic fractionation of these elements across taxa and tissue types is limited in comparison to the data available for N isotopes15,16.
Enamel, bone, and dentin—the three phosphatic hard tissues most often preserved in the fossil record—are composed of an inorganic bioapatite fraction (hydrated calcium phosphate mineral with structural and adsorbed carbonate ions; Ca10(PO4)6(OH)2)) and an organic matrix (i.e., proteins and lipids that vary in composition depending on the tissue)17,18,19,20. Relative to enamel, bone and dentin are poorly mineralized (~60 to 70% wt.), with smaller bioapatite crystals and a higher organic fraction (30%) made up predominantly of collagen. The organic matter in bone and dentin is, as a result, susceptible to diagenetic alteration, particularly in unfavorable depositional contexts (e.g., humid environments and acidic soils). As such, reliable bulk or compound-specific δ15N values are rare for fossil material older than tens of thousands of years21,22,23,24. In contrast, tooth enamel is more resistant to diagenetic alteration and preserves well across long (e.g., million-year) timescales20,25,26,27,28,29,30. The high-density, crystalline structure of mature tooth enamel (~85 to 95% wt.20) effectively encloses and protects organic matter within the biomineral matrix itself. Until recently, however, efforts to measure nitrogen isotopes in the organic matter of tooth enamel have been hampered by the low-N content of enamel (~0.5 to 2% wt. depending on taxon20,27,30,31) and the concomitantly large sample size that would be required for isotope analysis via traditional combustion with elemental analyzer isotope ratio mass spectrometry (EA-IRMS). While a modified EA-IRMS system known as a nano-EA has been developed32, analysis of enamel-bound nitrogen isotopes using this system have not been reported. More generally, sample size requirements for the nano-EA-IRMS method are still prohibitive for many applications (i.e., sampling of small and/or precious fossil teeth), and analytical precision remains relatively low. The oxidation-denitrification method33 drastically improves analytical precision from ~1.0‰ 2σ standard deviation for nano-EA measurements at 25 nmol of N32,34 to <0.2‰ at 5 nmol of N. Moreover, the rigorous pre-treatment protocol of the oxidation-denitrification method, conducted in a dedicated low-N clean lab, ensures that only endogenous, enamel-bound nitrogen is measured with low N blanks35.
Leichliter et al.35 measured nitrogen isotopes of organic matter in the tooth enamel (δ15Nenamel) of rodents from a controlled feeding experiment using the oxidation-denitrification method and established that δ15Nenamel records the nitrogen isotope composition of the diet. In the study, experimental animals that received plant-based diets had significantly lower δ15Nenamel values than those fed meat-based diets. Subsequently, Lüdecke and Leichliter et al.36 showed that the δ15Nenamel values of African mammals living in a single, well-constrained natural ecosystem also reflect trophic level differences (i.e., carnivore δ15Nenamel values were 4.0‰ higher, on average, than those of herbivores). However, the study was limited to a single ecosystem (Gorongosa National Park in Mozambique) and mammalian carnivores were poorly represented (n = 2). Moreover, δ15N values were measured for tooth enamel only and thus could not be directly compared to δ15N values of collagen (or any other tissue) from the same individuals. To further establish δ15Nenamel as a trophic level proxy and as a new tool for reconstructing ancient food webs, additional data from both modern and fossil fauna from natural ecosystems are needed.
Here we evaluate whether δ15Nenamel records dietary and trophic level information in modern mammals (n = 54) from several different ecosystems across Africa (Fig. S1, Table 1, and S1). Herbivores, including browsers (consuming mainly C3-plants), grazers (consuming mainly C4-plants), and mixed feeders (consuming both C3- and C4-plants), as well as omnivores and carnivores, were selected to ensure that different trophic levels and feeding behaviors were represented. In addition, paired δ15Nbone-collagen values were measured for a subset (n = 33) of the same individuals to evaluate whether enamel and bone collagen (the material in which δ15N has most often been measured in archeological and fossil contexts37) record similar dietary information.
Recent evidence from Martínez-García et al.38 demonstrates that the δ15N values and N contents of modern and fossil tooth enamel remain stable under experimentally induced conditions that favor organic matter degradation, including oxidative attack, partial dissolution, and heating. Taken together, these observations suggest that enamel-bound organic matter is highly protected and therefore has strong potential to preserve in vivo nitrogen isotope compositions even in diagenetically active settings. Further evidence for this comes from the work of Kast et al.39, who successfully reconstructed the trophic behavior of extinct megatooth sharks across the Cenozoic (~66 to 3.5 Ma) using δ15N values obtained from analysis of the enameloid of fossil shark teeth. It remains an open question, however, if fossil mammalian enamel preserves diet and trophic level information in a similar manner to the enameloid of shark teeth, given both the differences in mineral structures between mammalian enamel (hydroxylapatite) and shark enameloid (fluorapatite) as well as the differences in depositional environments. To date, tooth enamel δ15N values have not been measured in fossil mammalian ecosystems.
Here we apply the oxidation-denitrification method to fossil mammalian teeth from a Late Pleistocene (38.4 to 13.4 ka) terrestrial assemblage from Tam Hay Marklot Cave (THM), Laos in southeast (SE) Asia (Fig. S2). Fossils from this site are characterized by excellent enamel preservation, but poor preservation of bone and dentin, thus precluding δ15N analysis of collagen for most samples40. Additionally, Bourgon et al.40 measured zinc isotopes in the same specimens to reconstruct the trophic level. As such, the THM fauna represents an ideal test for the application of δ15Nenamel to the fossil record. We evaluate whether fossil enamel N content is in the same range as modern tooth enamel, consistent with good enamel-bound organic matter preservation, and assess whether the δ15Nenamel values of fossil mammalian teeth preserve dietary and trophic level information in a setting where the degradation of collagen prevents analysis of δ15Ncollagen.
Results
δ15Nenamel and δ13Cenamel were measured in modern (n = 54) and fossil (n = 10) teeth (primarily the latest forming molars from adult individuals). In addition, stable isotope values from mandibular collagen (δ15Nbone-collagen and δ13Cbone-collagen) were measured in a subset (n = 33) of the modern African fauna. Fossil dentin δ15Ndentin-collagen and δ13Cdentin-collagen values (n = 4) are from Bourgon et al.40. Isotopic data for all samples are reported in Tables 1, 2 and in Supplementary Data 1, 2.
Modern African mammals
Nitrogen isotopes
δ15Nenamel values of modern African mammals ranged from 3.5 to 14.9‰ (n = 54) (Fig. 1a, c and Table 1) and differ significantly according to diet (F(2,51) = 26.05, p < 0.001). Mean δ15Nenamel values were lowest in herbivores (6.2 ± 1.6‰; n = 36) and highest in carnivores (9.9 ± 2.0‰; n = 14), while omnivores had intermediate values (7.4 ± 0.4‰; n = 4). Herbivores and carnivores differed significantly (p < 0.001). Differences were also observed between herbivores with different feeding behaviors (i.e., browsing, grazing, and mixed feeding) and the other dietary groups (F(4,49) = 15.18, p < 0.001). Across ecosystems, browsing taxa typically had the lowest δ15Nenamel values (5.8 ± 1.0‰; n = 14), followed by grazing (6.1 ± 1.7‰; n = 15) and then mixed feeding taxa (7.5 ± 1.7‰; n = 7; Fig. 1). All herbivore dietary groups differed significantly from carnivores (p < 0.001 for both grazers and browsers; p = 0.015 for mixed feeders).
a δ15Nenamel and b δ15Nbone-collagen values for all modern African herbivores and carnivores measured in this study, with mean and standard deviation (1σ) indicated. The bolded black arrow illustrates the average trophic enrichment between herbivores and carnivores, which is similar in both datasets. Box plots of c δ15Nenamel and d δ15Nbone-collagen values for all dietary groups indicating the median and 25th percentiles. The δ15N values for all individuals are plotted by taxon within each dietary group (see Figure legend).
Δ15Nbone-collagen values ranged from 4.1 to 12.6‰ (n = 33) (Fig. 1b, d and Table 1) and differed significantly according to diet (F(3,29) = 11.66, p < 0.001). As in enamel, mean δ15Nbone-collagen values were lowest in herbivores (6.7 ± 2.0‰; n = 21) and highest in carnivores (9.8 ± 1.4‰; n = 12), and herbivores and carnivores differed significantly (p < 0.001). Among herbivores, δ15Nbone-collagen values were similar to δ15Nenamel values. Browsers had the lowest values (6.0 ± 1.2‰; n = 6), followed by grazers (6.4 ± 1.9‰; n = 12), and mixed feeders (9.3 ± 2.6‰; n = 3). Grazers and browsers differed significantly from carnivores (p < 0.001), but, in contrast to the enamel dataset, the mixed feeders did not differ from carnivores (p = 0.95).
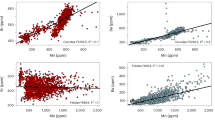
We found a significant, positive correlation between δ15Nenamel and δ15Nbone-collagen values (Pearson’s correlation r (31) = 0.865, p < 0.001; Fig. 2). An ordinary least squares regression yielded the following relationship: δ15Nenamel ~0.88 [95% confidence interval (CI): 0.66 to 1.1] × δ15Nbone-collagen + 0.43‰ [95% confidence interval (CI): −0.8 to 2.0] with no consistent, directional offset in δ15N values.
The dashed line represents a hypothetical 1:1 relationship between bone collagen and enamel values. The solid line indicates the regression, with a bootstrapped 95% confidence interval illustrated with gray shading. The equation for the regression, R2 value, and p value are indicated in the lower right of the figure. δ15Nenamel versus δ15Ndentin-collagen values for the four fossil Tam Hay Marklot (THM) specimens (see Table 2) are also plotted for reference (black diamonds) but were not included in the regression calculation.
Trophic enrichment between herbivores and carnivores (Δ15N = δ15Ncarnivore – δ15Nherbivore) is apparent in both datasets but is larger in enamel (3.7‰) compared to bone collagen (3.1‰) (Fig. 1).
Carbon isotopes
δ13Cenamel values ranged from −15.9 to +1.9‰ (n = 54; Fig. 3 and Table 1) and differed significantly according to diet (χ2(4) = 41, p < 0.001). Mean δ13Cenamel values were −7.3 ± 6.0‰ for herbivores (n = 36), and −6.8 ± 3.5‰ for carnivores (n = 14). Browsers had the lowest δ13Cenamel values (−13.0 ± 1.8‰; n = 14), followed by mixed feeders (−9.3 ± 3.3‰; n = 7) and then grazers (−1.0 ± 2.5‰; n = 15). Omnivores (−8.9 ± 1.5‰; n = 4) had intermediate values. Carbonate content was typically 4 to 7% wt.
Browsing (dark green), grazing (light green), and mixed feeding (brown-green) herbivores, omnivores (purple), and carnivores (blue) are indicated with color and taxa are indicated with symbols. Dashed lines for convex hulls represent the full range of variation, and shaded ellipses indicate 40% of estimated standard ellipse areas (SEAC). Herbivores and omnivores have relatively low δ15Nenamel values and generally plot in the lower half of the figure, while carnivores have higher δ15Nenamel values and plot in the top half of the figure. Green shaded bars at the top of the figure indicate tooth enamel δ13C isotopic ranges for modern herbivores corresponding to pure C3 (browsing) and C4 (grazing) resource utilization (after Cerling et al.104 and corrected for the fossil-fuel-induced shift in the δ13C of atmospheric CO2 for the period of 1950–1970).
δ13Cbone-collagen values ranged from −22.2 to −5.5‰ (n = 33; Fig. S4). Mean δ13Cbone-collagen values were −13.9 ± 6.3‰ (n = 21) for herbivores and −11.2 ± 4.3‰ (n = 12) for carnivores. Browsers and mixed feeders had low δ13Cbone-collagen values (−21.0 ± 0.9‰; n = 6 and −20.4 ± 1.4‰; n = 3, respectively), and grazers had significantly higher ones (−8.7 ± 1.7‰; n = 12).
δ13Cenamel was positively correlated with δ13Cbone-collagen (Spearman’s correlation Rs(31) = 0.867, p < 0.001). The positive correlation between δ13Cenamel and δ13Cbone-collagen was stronger within each dietary group (Spearman’s correlation Rs(19) = 0.893, p < 0.001 for herbivores and Rs(10) = 0.937, p < 0.001 for carnivores, respectively; see Fig. S4). The offset between δ13Cenamel and δ13Cbone-collagen was higher (8.2‰) in herbivores than in carnivores (4.5‰) as anticipated (see Supplementary Discussion).
Fossil mammals
Nitrogen content
The nitrogen content of modern African mammalian enamel was typically 2 to 10 nmol/mg (\(\bar{{{{{{\rm{x}}}}}}}\) = 4.8 ± 2.0 nmol/mg; n = 54) after reductive-oxidative cleaning (see Methods). Fossil tooth enamel N content was between 4 and 10 nmol/mg (\(\bar{{{{{{\rm{x}}}}}}}\) =6.4 ± 1.6 nmol/mg; n = 10) after cleaning, thus falling within the range of the modern enamel samples (Fig. 4 and Tables 1,2). No differences were observed between dietary or taxonomic groups in either modern or fossil datasets (Fig. S3), and, importantly, no significant correlation was observed between δ15Nenamel and N content (Pearson’s correlation r (62) = 0.0033, p = 0.979).
Nitrogen isotopes
The Late Pleistocene mammals from SE Asia exhibited a range of δ15Nenamel values (2.6 to 11.1‰, n = 10) similar to those of the modern African mammals. When added to the regression of the modern African mammal δ15Nbone-collagen and δ15Nenamel values, the four fossil specimens show a moderate, positive correlation, and—although this relationship is not significant (Kendall’s Tau rτ = 0.667, p = 0.333)—the regression residuals are mostly within the range of modern mammals (Fig. 2 and S7). As observed in the modern dataset, δ15Nenamel values were lowest in the herbivores (6.1 ± 2.7‰; n = 5), highest in the carnivore (a leopard, Panthera pardus; 11.1‰), and intermediate in the omnivores (7.3 ± 2.4‰; n = 4; Fig. 5). The grazers, including an Asian water buffalo (Bubalus bubalis) and an unspecified bovine (Bos sp.), had the lowest values (4.0 and 2.6‰, respectively), while the mixed-feeding cervid (Muntiacus muntjak; 7.6‰), the browsing rhinoceros (Rhinoceros sondaicus; 7.5‰), and the goat-like Sumatran serow (Capricornis sumatraensis; 8.9‰) all had higher values. Among the omnivores, the two pigs (Sus scrofa; 5.4 ± 0.6‰) had the lowest values, while the macaque (Macaca sp.; 9.9‰) and the Asian black bear (Ursus thibetanus; 8.8‰) had higher values. Overall, the range of δ15Nenamel values for omnivores encompasses that of herbivores, and thus there are no significant differences between these two diet groups (χ2(2) = 2.9, p = 0.235).
Herbivore (browsers, dark green; grazers, light green; mixed feeder, brown-green), omnivore (purple), and carnivore (blue) taxa are indicated. One individual was measured for all taxa, with the exception of the wild boar. Green shaded bars at the top of the figure indicate tooth enamel δ13C isotopic ranges for herbivores corresponding to pure C3 (browsing) and C4 (grazing) resource utilization (enamel δ13C after Cerling et al.104 and corrected for a fossil-fuel-induced shift in the δ13C atmospheric CO2 of ~ −2.0‰98). Grazing taxa are clearly distinguished from the rest of the fauna by their higher δ13Cenamel values. Most of the THM fauna lived and foraged in predominantly C3 habitats. Herbivores and omnivores have lower δ15Nenamel values compared to the single carnivore, which has the highest value (Panthera pardus).
Paired δ15Nenamel and δ66Znenamel values (data from Bourgon et al.40) for Tam Hay Marklot (n = 10) are negatively correlated (Pearson’s correlation r(8) = 0.671, p = 0.034; Fig. 6).
Carbon isotopes
Fossil δ13Cenamel values ranged from −15.7 to −0.8‰ (Fig. 5 and see Fig. S5). δ13Cenamel values were the lowest in the rhinoceros (−15.7‰) and serow (−15.2‰). The mixed-feeding muntjac had an intermediate value of −12.9‰. The bovine (indet.) and Asian water buffalo, both grazers, had the two highest values (−1.5 and −0.8‰, respectively). The suids, macaque, bear, and leopard all had similar δ13Cenamel values, ranging between −14.3 and −13.3‰ (see Table 2). As with modern mammals, carbonate content in the fossil enamel samples was typically between 4 and 7% wt.
Only four THM specimens were sufficiently preserved for δ13Cdentin-collagen analysis (data from Bourgon et al.40), but they show a positive relationship with δ13Cenamel data from the same specimens, consistent with the regression obtained for modern specimens (Fig. S4).
Discussion
Tooth enamel nitrogen and carbon isotopes clearly record diet and trophic level in modern African mammals. We observed an average difference in δ15Nenamel of 3.7‰ between herbivores and carnivores (Fig. 1a). This agrees well with the average trophic enrichment of ~3.5‰ in δ15N documented in numerous large-scale ecological studies5,10,11. Moreover, the observed δ15Nenamel values of the different dietary groups—specifically the low values of browsers and grazers, intermediate values of mixed feeders, and high values of carnivores (Fig. 1c)—agree well with published δ15N data for bone collagen6,7,8,41,42.
Previous research has shown that both abiotic (e.g., aridity, altitude, and soil chemistry) and biotic (e.g., digestive physiology, protein intake, and water-dependence) factors can cause variation in δ15N between different habitats and within trophic levels, sometimes to such a degree that the overall trophic level effect in nitrogen isotope ratios is obscured8,41,43,44,45,46,47. Although the samples included in this study are drawn from multiple localities across Africa (see Fig. S1, Table 1, and Table S1), trophic level patterns are nonetheless clearly discernible in the δ15Nenamel values of animals belonging to different dietary groups. Thus, while regional differences between sampling localities (i.e., baseline variation) may have contributed to observed intra-group variation in δ15Nenamel values, their effects do not obscure the overall trophic level effect recorded in δ15Nenamel.
Additionally, the same individuals’ paired δ15Nenamel and mandibular δ15Nbone-collagen values are positively correlated (Fig. 2), confirming that, when collagen is well preserved, enamel and collagen record very similar isotopic information. This finding is important because collagen is the most frequently measured material in nitrogen-based paleodietary studies, and diet-related nitrogen isotope fractionation in bone collagen in African ecosystems is well-understood4,5,6. Thus, demonstrating a clear link between δ15Nenamel and δ15Nbone-collagen represents an important step in establishing δ15Nenamel as a new geochemical approach for reconstructing past diets of fossil vertebrates.
The correlation between bone collagen and enamel δ15N values is not expected to be perfect because there are differences both in when these two hard tissues form as well as in the composition of their organic matter. Bone is a living, dynamic, and actively-growing tissue, which turns over on a time scale of multiple years, incorporating the isotopic composition of an animal’s diet throughout its lifetime48. In contrast, mammalian teeth mineralize during a discrete period relatively early in an animal’s life (i.e., weeks to months or even years, depending on taxon and tooth type and size49), after which the enamel becomes metabolically inactive and hence isotopically inert50. Thus, the two hard tissues represent different periods in an animal’s lifetime and may potentially record different diets; the isotopic composition of mandibular bone usually records the last three to five years before death48, whereas tooth enamel reflects discrete periods ranging from infancy to juvenile stages, to early adulthood, depending on the tooth type under consideration49. In order to capture the adult diet, and avoid enrichment in 15N as a result of the consumption of breastmilk51, we targeted the latest forming tooth in each taxon (typically a molar; see Tables 1, 2).
In addition to different tissue formation times, bone and enamel also differ in the composition of their organic matter. While the organic fraction of bone consists mainly of collagen (90%), the organic matter in enamel is comprised of enamel-specific proteins (predominantly amelogenin) and proteases27,52,53,54. There should thus not necessarily be a perfect 1:1 correlation between δ15Nenamel and δ15Nbone-collagen. Nevertheless, the positive correlation between enamel and bone collagen from the same individuals and the clear enrichment in δ15Nenamel across trophic levels confirm that δ15Nenamel records diet and trophic level in a manner similar to δ15Nbone-collagen.
Importantly, our dataset also includes measurements of nitrogen and carbon isotope values using the same aliquot of tooth enamel. δ15Nenamel and δ13Cenamel values are plotted together in Fig. 3 and reveal clear, interpretable patterns that are in good agreement with expected dietary habits for the modern taxa included in this study. Specific results for herbivores, omnivores, and carnivores are discussed in the following text.
While herbivores have a lower overall mean δ15Nenamel value than carnivores, they exhibit a high degree of variation in δ15Nenamel as a dietary group. Numerous factors have been proposed to drive this variation. Abiotic and biotic variables, including environment (e.g., precipitation, temperature, soil chemistry, and their effect on soil N cycling), physiology (e.g., water conservation and digestive physiology46,47), and diet (e.g., feeding strategy, foraging microhabitat, and macronutrient composition of diet)8,41,43,55,56,57,58,59,60,61 can all influence δ15N. For instance, consumed plant part (e.g., leaf, fruit, stem, and root) and plant nutritional quality (e.g., protein-, fiber-, and fat-content), as well as the digestive anatomy (ruminant vs. non-ruminant) and the nutritional status (e.g., starvation, pregnancy, and lactation) of the animal itself, also can impact herbivore δ15N values47,57,58,59,60,61,62,63,64,65. Ultimately, the δ15Nenamel values of herbivore body tissues are driven primarily by the isotopic composition of the plants they consume, which is in turn controlled by abiotic factors specific to each ecosystem55 (see Fig. S6 and Supplementary Discussion for more details).
When grouped according to feeding behavior, the mixed feeders stand out in particular amongst the herbivores. While browsing and grazing taxa do not differ in δ15Nenamel, mixed feeders (springbok, impala, and elephants) had significantly higher δ15Nenamel values, even overlapping with those of the carnivores in some cases (Fig. 1c). Despite this overlap, mixed feeders still differ significantly from carnivores in δ15Nenamel. This is not the case for δ15Nbone-collagen, although this difference may have been driven, at least in part, by the smaller size of the collagen dataset.
These herbivore δ15N values are consistent with previously published herbivore data from modern ecosystems6,8,25,36,41,42,43,61,66. Ambrose41 observed relatively high δ15Nbone-collagen values (also overlapping with those of co-occurring carnivores) in mixed-feeding herbivores from eastern Africa, Sealy et al.8 observed higher δ15Nbone-collagen values in mixed-feeding springbok compared to other herbivorous taxa in southern Africa, and Codron et al.42 measured the highest herbivore δ15N values in the feces of mixed feeders (impala and nyala) in their study of herbivores in Kruger National Park. African elephant tissue δ15N values vary widely, depending on which region of Africa the animals inhabit, but our observed δ15Nenamel values (5.4 to 10.0‰) fit well within the large range of published bone collagen values (2.0 to 16.0‰) for this taxon8,67,68,69. There is much debate regarding the possible mechanisms driving elevated values in mixed feeders and it remains unclear why mixed feeders tend to have higher δ15N values than other herbivores overall (see Fig. S6 and Supplementary Discussion for a more detailed discussion of this topic). Our δ15Nenamel dataset for one given locality in Africa is too small to test if this holds true for tooth enamel amongst mixed feeders more broadly. Regardless the overall pattern observed in our dataset is consistent with data published for herbivores from African and South American ecosystems36,61,66.
For the omnivores in this study, we analyzed enamel from baboons (Papio), which are of particular interest for the potential application of this method to questions about human evolution, as baboons live in open savanna ecosystems and have been proposed as a model taxon for early hominins. Baboons are ecological and dietary generalists that consume a wide variety of foods in an opportunistic manner70,71,72,73, including many types of plants, as well as insects, small animals, eggs, etc. However, although baboons are considered dietary generalists, their diet often consists primarily of plant matter74.
The δ15Nenamel values we obtained for baboons are consistent with existing stable isotope data for these primates62. Baboon δ15Nenamel values were significantly lower than those of carnivores and were instead comparable to those of herbivores, specifically the mixed feeders (Fig. 1). Although some studies have found that baboons have low δ15N values compared to sympatric herbivores6,62, perhaps related to the consumption of N2-fixing plants and/or underground storage organs, no clear tendency towards lower values was observed in baboon δ15Nenamel values compared to herbivores in our dataset. The teeth sampled for this study would have formed just at the end of the juvenile period, and young, low-ranking baboons rarely consume meat, a behavior that has primarily been observed in dominant adult males36,75,76. Thus the relatively low δ15Nenamel values for the baboons are expected.
δ15Nenamel values were the highest for the carnivores, evidencing clear trophic enrichment compared to herbivores and falling into the range of δ15Nbone-collagen values typical for carnivores. Within the carnivore guild, we also observed differences in δ15Nenamel between taxa. Leopards (Panthera pardus) and wild dogs (Lycaon pictus), for instance, had lower δ15Nenamel values than spotted hyenas (Crocuta crocuta) and lions (Panthera leo). Overall, the δ15Nenamel values of spotted hyenas are higher than those of other carnivore species, and, in two individuals, substantially higher than all other δ15N values measured for this species in this study. It is probable that these higher values incorporate a nursing signal, as the permanent dentition of spotted hyenas mineralizes early and erupts at approximately the same time that weaning occurs77. If these two outliers are excluded from the carnivore δ15Nenamel dataset, Δ15Ncarnivore-herbivore decreases to 3.1 ‰, but trophic enrichment between herbivores and carnivores nevertheless remains significant and equal to that of δ15Nbone-collagen.
Although the observed differences between carnivore δ15Nenamel values are potentially related to niche separation and differential habitat use/prey preference, no strong conclusions can be drawn from the differences in δ15Nenamel values alone, especially when considering that these samples are drawn from disparate localities. However, carbon isotope data help shed further light on niche separation between carnivore taxa. For instance, while all carnivores included in this study are typical savanna dwellers, δ13Cenamel values for leopards and wild dogs in this dataset indicate that these two taxa relied more heavily on browsing prey (i.e., C3-consuming prey) compared to spotted hyenas and lions (Fig. 3). Most carnivores selectively hunt specific taxa and their diets can be strongly influenced by competition with other carnivores. Leopards, for example, prefer relatively small prey (e.g., body mass 10 to 40 kg), which occur in dense habitats; for example, impala, bushbuck, and common duiker, while larger prey and species restricted to open vegetation are generally avoided78. Similarly, wild dogs tend to hunt in areas of denser vegetation and target browsing and mixed-feeding herbivores in regions where they co-occur with hyenas and lions79. Lions and hyenas, in contrast, have higher δ13Cenamel values, consistent with the consumption of a greater proportion of grazing taxa (i.e., C4-consuming prey) which occupy more open environments. These carnivore data illustrate the potential to refine reconstructions of trophic niches using combined δ15Nenamel and δ13Cenamel analyses.
Importantly, this study was not designed to explicitly test the effect of the potential confounding abiotic or biotic factors that may be driving variation in herbivore/carnivore δ15N values, and our δ15Nenamel dataset for any one given locality in Africa is too small to do so properly. Rather, we set out to demonstrate that δ15Nenamel values record diet in a robust manner comparable to other commonly measured tissue types, especially collagen, the material most frequently measured in the archeological and fossil record. Our data conclusively demonstrate that tooth enamel organic matter records dietary information, confirming the utility of δ15Nenamel as a trophic level proxy.
Tooth enamel isotopes measured in Late Pleistocene fossil teeth indicate good preservation of enamel-bound organic matter and record diet and trophic level information. In their study of zinc isotopes in fossil tooth enamel from THM, Bourgon et al.40 attempted to extract collagen from dentin for δ13C and δ15N analysis. Of 72 samples, 23 had enough dentin to attempt collagen extraction, but only four specimens had sufficiently preserved collagen (i.e., with a C:N between 2.9 and 3.614,80) for stable isotope analysis. Collagen yield was also relatively poor (<1% for all samples). Thus, reconstruction of trophic levels based δ15Ncollagen values was not feasible for this assemblage. In contrast, all available THM fossil teeth analyzed using the oxidation-denitrification method (n = 10) have nitrogen contents that are comparable to modern tooth enamel (e.g., Leichliter et al.36, Lüdecke and Leichliter et al.35, this study; Fig. 4). If additional exogenous N would have been added during fossilization, we would expect fossil samples to have a higher N content than their modern counterparts. Alternatively, if the organic matter was degraded over time, we would expect a clear decrease in N content with respect to modern samples38,81. Either scenario, i.e., the addition of exogenous N or degradation of endogenous organic matter, could result in corresponding directional changes in δ15Nenamel values in relation to N content. However, the N content and δ15Nenamel values of the fossil samples are within the range of modern specimens and show no correlation (see Fig. 4), indicating good enamel-bound organic matter preservation in our fossil dataset. These observations are consistent with the results of laboratory degradation experiments38 and with measurements of million-year-old marine microfossils39,82,83, which suggest that biomineral structures act as an effective physical barrier that protects organic matter from degradation. Although it is a small dataset, the paired δ15Nenamel and δ15Ndentin-collagen values for the four fossil specimens are also positively, though not significantly, correlated (see Fig. 2 and Fig. S7).
Within the THM assemblage, fossil δ15Nenamel values record trophic enrichment in 15N (i.e., low values for herbivores, intermediate values for omnivores, and a high value for the carnivore; Fig. 5). While the current fossil dataset is too small to draw any strong conclusions regarding the trophic structure of the THM fossil assemblage, these patterns corroborate the good preservation of dietary N-isotope compositions in tooth enamel for samples in which the collagen is already degraded. Additionally, we observed some interesting results. For example, the δ15Nenamel values of the browsers were relatively high compared to those of the grazers, deviating from the pattern of low δ15Nenamel values observed for browsers in the modern African fauna. African herbivores living in forests generally exhibit lower δ15Ncollagen values than herbivores from more open (i.e., grassland) environments6,41. It is plausible that the relatively higher δ15Nenamel values of the browsers from THM may be unique to this region and time period, or reflect selective feeding behavior, as plant δ15N values are known to vary geographically as well as according to plant taxon, part, and position in the forest canopy84,85 however, it is difficult to discern any cogent pattern with only a few individuals in each feeding category. The δ13Cenamel values reveal that, except for the two grazers, the analyzed fossil taxa from THM lived and foraged in predominantly C3 environments. This is consistent with the ecology of the fauna present in the assemblage based on taxonomy and dental morphology, and with what is known about the environment in this region of Southeast Asia during the Late Pleistocene, which was probably predominantly forested, albeit not as densely as a closed low-light tropical rainforest40,86,87.
δ15Nenamel and δ66Znenamel values from the same individuals show a negative correlation (Fig. 6). This is expected as δ66Znenamel has been shown to decrease with increasing trophic level40,88,89,90. Encouragingly, reconstructed trophic positions for omnivorous taxa based on the two isotope systems are in good agreement. Specifically, δ15Nenamel value(s) for the pigs are low, the bear is intermediate, and the macaque is high, while the inverse is true for δ66Znenamel. This finding is especially promising given the difficulty of interpreting variations in bulk tissue δ15N isotope values (such as those obtained from e.g., enamel organic matter and collagen) for taxa with generalist feeding strategies like omnivores and mixed feeders. Future studies that incorporate both δ15Nenamel and δ66Znenamel (and other isotope systems) may help us better resolve omnivores’ dietary behavior, a task that is particularly challenging given their broad resource use.
In this study, we present paired organic nitrogen and inorganic carbon isotopic values measured in a single aliquot (5 to 7 mg) of tooth enamel. Importantly, our study demonstrates that the δ15Nenamel values of mammals from natural ecosystems record diet and trophic behavior in the same manner as the classical dietary proxy of δ15Ncollagen. The δ15Nenamel values of carnivores are elevated by 3 to 5‰ compared to those of herbivores and omnivores in both modern and fossil food webs, which is comparable to the well-established 3 to 4‰ enrichment in δ15N per trophic level along food chains3,4,5,6,7,8,9,10. Furthermore, paired δ15Nenamel and δ15Nbone-collagen values from the same individuals are positively correlated. While more studies comparing bone, dentin, and enamel δ15N from the same individual will be useful in characterizing the exact nature of the relationship between these different tissue types, the results of these analyses conclusively demonstrate that δ15Nenamel and mandibular δ15Nbone-collagen record similar diet and trophic information. Indeed, given what is known about the inherent variability in δ15N related to abiotic and biotic factors, the coherence of our modern dataset and the consistency of the enrichment in 15N between trophic levels in samples drawn from disparate localities is remarkable, and demonstrates that δ15Nenamel is a robust trophic proxy with great potential for application in paleodietary studies.
The results of our analysis of δ15Nenamel of enamel samples from modern and fossil teeth are highly promising. In the archeological and paleontological record, the degradation of organic matter (specifically collagen) is a fundamental limitation91 restricting measurements to relatively young, well-preserved samples. Therefore, the ability to measure the nitrogen isotope composition of the organic matter preserved in diagenetically robust tooth enamel (for example, at Tam Hay Marklot Cave) has the potential to be used to investigate the trophic ecology of ancient or even extinct animals over time periods far beyond the limit of collagen preservation. For example, dental wear, stone tools, and cut marks on fossil bones associated with early hominins suggest that members of the genus Australopithecus may have engaged in meat consumption as early as 3 million years ago92. However, no direct geochemical data exists to evaluate this claim, and the inference that Australopithecus shaped and used tools to access animal resources prior to the emergence of Homo is heavily debated93. δ15Nenamel values of early hominins and associated fauna have the potential to shed new light on this debate by providing geochemical evidence for the onset and intensification of animal-resource consumption throughout human evolution.
Methods
Experimental design
Tooth enamel (n = 54) and mandibular bone (n = 33) were sampled from 20 modern African mammalian taxa housed in the zoological collection at the University of Hamburg, Germany. We targeted herbivores (including browsers, grazers, and mixed feeders), omnivores, and carnivores to evaluate the effect of trophic level on δ15Ν values. A minimum of three individuals were sampled per taxon. Enamel from the last forming tooth was preferentially used whenever possible to avoid the effect of milk consumption (which typically results in ~2 to 3‰ higher δ15N values in the tissues of nursing individuals compared to the mother’s tissues51). Specimens were sampled using a hand-held Dremel with a diamond burr tip. For fossil specimens (n = 10; 9 taxa) from THM, a chip of tooth enamel was crushed and ground to a fine powder in an agate mortar and pestle. All data and associated information are presented in Tables 1, 2, Supplementary Tables S1–4, and Supplementary Data 1–4.
Tooth enamel nitrogen isotope measurement
Tooth enamel samples (5 to 7 mg) were measured for δ15Nenamel in nine analytical batches using the oxidation-denitrification method. δ15Nenamel values of bacterially converted N2O were measured via gas chromatography-isotope ratio mass spectrometry at the Max Planck Institute for Chemistry (MPIC, Mainz, Germany). The method used to measure δ15N values of tooth enamel is described only briefly here; for a detailed description, see Leichliter et al.35 and references therein. The procedure consists of four main steps: (1) 5 to 7 mg of tooth enamel powder is subjected to a reductive-oxidative cleaning to remove exogenous organic matter94; (2) samples are demineralized, and all endogenous organic matter (i.e., intra-and inter-crystalline bound N) is oxidized to nitrate using a persulfate oxidizing reagent (0.67–0.70 g of four times re-crystallized potassium persulfate added to 4 ml of 6.25 N NaOH solution in 95 ml Milli-Q water)33, (3) nitrate is quantitatively converted to N2O via the ‘denitrifier’ method95, and (4) sample-derived N2O is extracted, and its nitrogen isotopic composition is measured on a custom system online to a Thermo ScientificTM 253 Plus isotope ratio mass spectrometer (IRMS). Isobaric interference with CO2, is minimized by two stages of cryo-isolation and gas chromatography columns as detailed in95,96. These automated steps result in the full separation of the CO2 peak from the N2O peak, which can be seen in each sample chromatogram. International materials (USGS 40, USGS 65, USGS 41, USGS 34, and IAEA-NO-3) and in-house standards (PO-1, LO-1, AG-Lox, Noto-1; see Leichliter et al.35 for details) are included in every run and each step of this process, allowing us to monitor measurement stability, and evaluate the possibility of matrix-based effects during cleaning. Blank N concentration and δ15N were measured for each analysis batch, and the sample N content and δ15N values were corrected using the blank measurements of the associated batch. Samples were measured in duplicate or triplicate (resulting in a total of 115 individual measurements) and in separate batches whenever possible. Blank N content was between 0.3 and 0.6 nmol/ml (\(\bar{{{{{{\rm{x}}}}}}}\) = 0.37 ± 0.11 nmol/ml), resulting in an average blank contribution of 3% or less. Inter-batch precision (± 1σ) in δ15N for in-house standards was 0.4‰ for coral standards (n = 43) and 0.4‰ for tooth enamel standards (n = 41) across all analysis batches (see Supplementary Data 3).
Tooth enamel carbon isotope measurement
Small aliquots (50–100 µg) of untreated enamel powder were measured using high-precision continuous-flow mass spectrometry. δ13Cenamel analyses were performed at the MPIC on a Thermo Delta-V continuous-flow mass spectrometer coupled to a Gasbench II gas preparation system, equipped with a liquid nitrogen cryogenic trap (i.e., the “cold trap method”)97. Untreated enamel powder (see Fig. S5) was reacted with >99% H3PO4 for 90 min at 70 °C before the resulting CO2 was introduced to the continuous flow system. Isotope data are calculated by direct comparison to eleven replicates of a tooth enamel standard (AG-Lox; δ13C = −11.46 ± 0.2‰) analyzed in each batch. Samples were analyzed in a total of seven analytical batches, and samples were measured in duplicate or triplicate (where possible) in different batches. A logarithmic fit through the isotope ratios versus peak size for the AG-Lox replicates was used to eliminate fractionation effects due to sample size. After these corrections, the reproducibility of international and in-house carbonate standards (IAEA-603, NBS 18, VICS), as well as sedimentary phosphate (NIST SRM 120c), was generally better than 0.3‰ (1σ) and <0.1‰ (1σ) within batches for AG-Lox (Supplementary Data 4). Sample δ13C values were corrected for changes in atmospheric δ13C where appropriate after Hare et al.98 and corrections are specified in the text and in the relevant figure captions. Carbonate contents were derived from standard versus sample total peak area ratios (7.5% structural carbonate content for AG-Lox; after Vonhof et al.97).
Bone collagen carbon and nitrogen isotope measurement
Bone collagen was extracted according to the protocol described by Richards and Hedges99. Up to 150 mg of mandibular bone powder was demineralized with 0.5 M HCl for at least 24 h at 4 °C. Samples were then centrifuged, the supernatant discarded, and the remaining collagen was rinsed three times with deionized water. Afterward, a pH of 2 to 3 was obtained by adding a few drops of 0.5 M hydrochloric acid. The sample was then gelatinized by heating collagen to 70 °C for 48 h. After 48 h, gelatinized collagen was filtered (using 0.55 µm pore size midi-filters), centrifuged, the supernatant was discarded, and the remaining collagen was freeze-dried. Collagen yield was between 5 and 25%.
δ13Cbone-collagen and δ15Nbone-collagen were measured in the same aliquot of collagen at the Institute for Organic Chemistry, Johannes Gutenberg University Mainz, Germany, using an IsoPrimeTM High-Performance Stable Isotope Ratio Mass Spectrometer, GV Instruments. Each run included the following standards: ten Sulfanilamide replicates, three replicates each of IAEA-N1, IAEA-N2, IAEA-CH6, and IAEA-CH7, and two replicates of the bovine liver (NIST SRM 1577). For collagen measurements, 1 to 2 mg of extracted collagen was analyzed in replicate for each specimen. Analytical precision was better than ± 0.25‰ (1σ) for both δ13C and δ15N.
Statistics and reproducibility
Isotopic datasets were evaluated to determine if the data were normally distributed and if the variance was equal between groups using Levene’s test. A non-parametric Kruska–Wallace test followed by a Dunn’s post hoc test with a Bonferroni correction for pairwise comparisons was used when the requirements for ANOVA were not satisfied; otherwise, analysis of variance (ANOVA; one-tailed) was used to identify statistically significant differences in isotopic values between groups for modern African fauna. Where ANOVA indicated statistical significance, pairwise comparisons were made using a Tukey–Kramer HSD post hoc test to determine which groups differed from one another in their isotope values. Statistically significant relationships between paired isotopic values from the same individuals were determined using Pearson’s correlation (two-tailed), Kendall Rank correlation, or Spearman Rank correlation (two-tailed) where appropriate. Statistical analyses were performed using Paleontological Statistics Version 4.09 (PAST4) and JMP®, Version 16 statistical software using an alpha level for the significance of 0.05. Detailed results of pairwise comparisons can be found in Tables S2–S4. Sample sizes and the number of replicates are stated in the Results section as well as in Supplementary Data 1–4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The Supplementary Materials include additional information regarding analytical procedures, data processing, sampling locations, and isotopic data for the samples included in this study. All isotope values are provided in the main text as well in an associated excel database (Supplementary Data 1–4).
References
Cerling, T. E. & Harris, J. Carbon isotope fractionation betwwen diet and bioapatite in ungulate mammals and implications for ecological and palaecological studies. Oecologia 120, 347–363 (1999).
Balter, V. et al. Ecological and physiological variability of Sr/Ca and Ba/Ca in mammals of West European mid-Würmian food webs. Palaeogeogr. Palaeoclimatol. Palaeoecol. 186, 127–143 (2002).
Schoeninger, M. J. Stable isotope analyses and the evolution of human diets. Annu. Rev. Anthropol. 43, 413–430 (2014).
Minagawa, M. & Wada, E. Stepwise enrichment of 15N along food chains: further evidence and the relation between 15N and animal age. Geochim. Cosmochim. Acta 48, 1135–1140 (1984).
Schoeninger, M. J. & DeNiro, M. J. Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim. Cosmochim. Acta 48, 625–639 (1984).
Ambrose, S. H. & DeNiro, M. J. The isotopic ecology of East-African mammals. Oecologia 69, 395–406 (1986).
Ambrose, S. H. Stable carbon and nitrogen isotope analysis of human and animal diet in Africa. J. Hum. Evol. 15, 707–731 (1986).
Sealy, J. C., van der Merwe, N. J., Thorp, J. A. L. & Lanham, J. L. Nitrogen isotopic ecology in southern Africa: implications for environmental and dietary tracing. Geochim. Cosmochim. Acta 51, 2707–2717 (1987).
DeNiro, M. J. & Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 45, 341–351 (1981).
Caut, S., Angulo, E. & Courchamp, F. Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 46, 443–453 (2009).
Bocherens, H. & Drucker, D. Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: case studies from recent and ancient terrestrial ecosystems. Int. J. Osteoarchaeol. 13, 46–53 (2003).
Britton, K., Gaudzinski-Windheuser, S., Roebroeks, W., Kindler, L. & Richards, M. P. Stable isotope analysis of well-preserved 120,000-year-old herbivore bone collagen from the Middle Palaeolithic site of Neumark-Nord 2, Germany reveals niche separation between bovids and equids. Palaeogeogr. Palaeoclimatol. Palaeoecol. 333-334, 168–177 (2012).
Jaouen, K. et al. Exceptionally high δ15N values in collagen single amino acids confirm Neandertals as high-trophic level carnivores. Proc. Natl Acad. Sci. USA 116, 4928–4933 (2019).
DeNiro, M. J. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 317, 806–809 (1985).
Jaouen, K. & Pons, M.-L. Potential of non-traditional isotope studies for bioarchaeology. Archaeol. Anthropol. Sci. 9, 1389–1404 (2017).
Martin, J., Tacail, T. & Balter, V. Non-traditional isotope perspectives in vertebrate palaeobiology. Palaeontology 60, 485–502 (2017).
Krueger, H. W. Exchange of carbon with biological apatite. J. Archaeol. Sci. 18, 355–361 (1991).
Driessens, F. C. M., van Dijk, J. W. E. & Borggreven, J. M. P. M. Biological calcium phosphates and their role in the physiology of bone and dental tissues I. Composition and solubility of calcium phosphates. Calcif. Tissue Res. 26, 127–137 (1978).
He, L. H. & Swain, M. V. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J. Mech. Behav. Biomed. Mater. 1, 18–29 (2008).
Teruel, Jd. D., Alcolea, A., Hernández, A. & Ruiz, A. J. O. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch. Oral. Biol. 60, 768–775 (2015).
Ostrom, P. H., Macko, S. A., Engel, M. H. & Russell, D. A. Assessment of trophic structure of Cretaceous communities based on stable nitrogen isotope analyses. Geology 21, 491–494 (1993).
Ostrom, P., Macko, S., Engel, M., Silfer, J. & Russell, D. Geochemical characterization of high molecular weight material isolated from Late Cretaceous fossils. Org. Geochem. 16, 1139–1144 (1990).
Palmqvist, P., Gröcke, D. R., Arribas, A. & Fariña, R. A. Paleoecological reconstruction of a lower Pleistocene large mammal community using biogeochemical (δ13C, δ15N, δ18O, Sr:Zn) and ecomorphological approaches. Paleobiology 29, 205–229 (2003).
Jones, A. M. et al. Biogeochemical data from well preserved 200 ka collagen and skeletal remains. Earth Planet. Sci. Lett. 193, 143–149 (2001).
Lee-Thorp, J. & der Merwe, Van. N. J. Carbon isotope analysis of fossil bone apatite. South Afr. J. Sci. 83, 712–715 (1987).
Wang, Y. & Cerling, T. E. A model of fossil tooth and bone diagenesis: implications for paleodiet reconstruction from stable isotopes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 107, 281–289 (1994).
Robinson, C., Kirkham, J., Brookes, S. J., Bonass, W. A. & Shore, R. C. The chemistry of enamel development. Int. J. Dev. Biol. 39, 145–152 (1995).
Koch, P. L., Tuross, N. & Fogel, M. L. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J. Archaeol. Sci. 24, 417–429 (1997).
Zazzo, A., Lécuyer, C., Sheppard, S. M. F., Grandjean, P. & Mariotti, A. Diagenesis and the reconstruction of paleoenvironments: A method to restore original δ18O values of carbonate and phosphate from fossil tooth enamel. Geochim. Cosmochim. Acta 68, 2245–2258 (2004).
Robinson, C. Enamel maturation: a brief background with implications for some enamel dysplasias. Front. Physiol. https://doi.org/10.3389/fphys.2014.00388 (2014).
Savory, A. & Brudevold, F. The distribution of nitrogen in human enamel. J. Dent. Res. 38, 436–442 (1959).
Polissar, P. J., Fulton, J. M., Junium, C. K., Turich, C. C. & Freeman, K. H. Measurement of 13C and 15N isotopic composition on nanomolar quantities of C and N. Anal. Chem. 81, 755–763 (2009).
Knapp, A. N., Sigman, D. M. & Lipschultz, F. N isotopic composition of dissolved organic nitrogen and nitrate at the Bermuda Atlantic Time-series study site. Glob. Biogeochem. Cycles 19, 1–15 (2005).
Fulton, J. M., Arthur, M. A., Thomas, B. & Freeman, K. H. Pigment carbon and nitrogen isotopic signatures in euxinic basins. Geobiology 16, 429–445 (2018).
Leichliter, J. N. et al. Nitrogen isotopes in tooth enamel record diet and trophic level enrichment: Results from a controlled feeding experiment. Chem. Geol. 563, 120047–120047 (2021).
Lüdecke, T. et al. Carbon, nitrogen, and oxygen stable isotopes in modern tooth enamel: a case study from Gorongosa National Park, central Mozambique. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2022.958032 (2022).
Lee-Thorp, J. On isotopes and old bones. Archaeometry 50, 925–950 (2008).
Martinez-Garcia, A. et al. Laboratory assessment of the impact of chemical oxidation, mineral dissolution, and heating on the nitrogen isotopic composition of fossil-bound organic matter. Geochem. Geophys. Geosyst. 23, e2022GC010396 (2022).
Kast, E. R. et al. Cenozoic megatooth sharks occupied extremely high trophic positions. Sci. Adv. 8, eabl6529 (2022).
Bourgon, N. et al. Zinc isotopes in Late Pleistocene fossil teeth from a Southeast Asian cave setting preserve paleodietary information. Proc. Natl Acad. Sci. USA 117, 4675–4681 (2020).
Ambrose, S. H. Effects of diet, climate and physiology on nitrogen isotope abundances in terrestrial foodwebs. J. Archaeol. Sci. 18, 293–317 (1991).
Codron, D., Codron, J., Sponheimer, M. & Clauss, M. Within-population isotopic niche variability in savanna mammals: disparity between carnivores and herbivores. Front. Ecol. Evol. 4, 15 (2016).
Heaton, T. H. E., Vogel, J. C., Von La Chevallerie, G. & Collett, G. Climatic influence on the isotopic composition of bone nitrogen. Nature 322, 822–823 (1986).
Amundson, R. et al. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 17, 5 (2003).
Männel, T., Auerswald, K. & Schnyder, H. Altitude gradients of grassland carbon and nitrogen isotope composition are recorded in hair of grazers. Glob. Ecol. Biogeogr. 16, 583–592 (2007).
Hartman, G. Are elevated δ15N values in herbivores in hot and arid environments caused by diet or animal physiology. Funct. Ecol. 25, 122–131 (2011).
Codron, J. et al. Taxonomic, anatomical, and spatio-temporal variations in the stable carbon and nitrogen isotopic compositions of plants from an African savanna. J. Archaeol. Sci. 32, 1757–1772 (2005).
Manolagas, S. C. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21, 115–137 (2000).
Ungar, P. S. Mammal Teeth: Origin, Evolution, and Diversity (Johns Hopkins Univ. Press, 2010).
Balasse, M., Bocherens, H. & Mariotti, A. Intra-bone variability of collagen and apatite isotopic composition used as evidence of a change of diet. J. Archaeol. Sci. 26, 593–598 (1999).
Fuller, B. T., Fuller, J. L., Harris, D. A. & Hedges, R. E. M. Detection of breastfeeding and weaning in modern human infants with carbon and nitrogen stable isotope ratios. Am. J. Phys. Anthropol. 129, 279–293 (2006).
Castiblanco, G. A. et al. Identification of proteins from human permanent erupted enamel. Eur. J. Oral. Sci. 123, 390–395 (2015).
Lacruz, R. S., Habelitz, S., Wright, J. T. & Paine, M. L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 97, 939–993 (2017).
Welker, F. et al. The dental proteome of Homo antecessor. Nature 580, 235–238 (2020).
Murphy, B. P. & Bowman, D. M. J. S. Kangaroo metabolism does not cause the relationship between bone collagen δ15N and water availability. Funct. Ecol. 20, 1062–1069 (2006).
Ambrose, S. H. & DeNiro, M. J. Reconstruction of African human diet using bone collagen carbon and nitrogen isotope ratios. Nature 319, 321–324 (1986).
Sponheimer, M. et al. Nitrogen isotopes in mammalian herbivores: Hair δ15N values from a controlled feeding study. Int. J. Osteoarchaeol. 13, 80–87 (2003).
Fuller, B. T. et al. Nitrogen balance and δ15N: why you’re not what you eat during pregnancy. Rapid Commun. Mass Spectrom. 18, 2889–2896 (2004).
Robbins, C. T., Felicetti, L. A. & Sponheimer, M. The effect of dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecologia 144, 534–540 (2005).
Cantalapiedra-Hijar, G. et al. Diet–animal fractionation of nitrogen stable isotopes reflects the efficiency of nitrogen assimilation in ruminants. Br. J. Nutr. 113, 1158–1169 (2015).
Tejada, J. V. et al. Comparative isotope ecology of western Amazonian rainforest mammals. Proc. Natl Acad. Sci. USA 117, 26263–26272 (2020).
Codron, D., Lee-Thorp, J. A., Sponheimer, M., de Ruiter, D. & Codron, J. Inter- and intrahabitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal δ13C, δ15N, and %N. Am. J. Phys. Anthropol. 129, 204–214 (2006).
Robinson, T. F. et al. Digestibility and nitrogen retention in llamas and goats fed alfalfa, C3 grass, and C4 grass hays. Small Rumin. Res. 64, 162–168 (2006).
Mekota, A. M., Grupe, G., Ufer, S. & Cuntz, U. Serial analysis of stable nitrogen and carbon isotopes in hair: monitoring starvation and recovery phases of patients suffering from anorexia nervosa. Rapid Commun. Mass Spectrom. 20, 1604–1610 (2006).
Reynard, L. & Tuross, N. The known, the unknown and the unknowable: weaning times from archaeological bones using nitrogen isotope ratios. J. Archaeol. Sci. 53, 618–625 (2015).
Ambrose, S. H. in Biogeochemical Approaches to Paleodietary Analysis (eds Katzenberg, M. A. & Ambrose, S. H.) Ch. 12 (Springer, 2002).
Vogel, J. C., Talma, S., Hall-Martin, A. J. & Viljoen, P. J. Carbon and nitrogen isotopes in elephants. South Afr. J. Sci. 86, 147–150 (1990).
Tieszen, L., Boutton, T., Ottichilo, W., Nelson, D. & Brandt, D. An assessment of long‐term food habits of Tsavo elephants based on stable carbon and nitrogen isotope ratios of bone collagen. Afr. J. Ecol. 27, 219–226 (2008).
Koch, P. L. et al. Isotopic tracking of change in diet and habitat use in african elephants. Science 267, 1340–1343 (1995).
Swedell, L. In Primates in Perspective (ed. Campell, C. J.) Ch. 15 (Oxford Univ. Press, 2011).
Altman, S. A. Foraging for Survival: Yearling Baboons in Africa (Univ. Chicago Press, 1998).
Byrne, R. W., Whiten, A., Henzi, S. P. & McCulloch, F. M. Nutritional constraints on mountain baboons (Papio ursinus): implications for baboon socioecology. Behav. Ecol. Sociobiol. 33, 233–246 (1993).
Barton, R. A., Whiten, A., Strum, S. C., Byrne, R. W. & Simpson, A. J. Habitat use and resource availability in baboons. Anim. Behav. 43, 831–844 (1992).
Hill, R. A. & Dunbar, R. I. M. Climatic determinants of diet and foraging behaviour in baboons. Evolut. Ecol. 16, 579–593 (2002).
Hamilton, W. J. & Busse, C. Social dominance and predatory behavior of chacma baboons. J. Hum. Evol. 11, 567–573 (1982).
Rhine, R. J., Norton, G. W., Wynn, G. M., Wynn, R. D. & Rhine, H. B. Insect and meat eating among infant and adult baboons (Papio cynocephalus) of Mikumi National Park, Tanzania. Am. J. Phys. Anthropol. 70, 105–118 (1986).
Binder, W. J. & Van Valkenburgh, B. Development of bite strength and feeding behaviour in juvenile spotted hyenas (Crocuta crocuta). J. Zool. 252, 273–283 (2000).
Hayward, M. W. et al. Prey preferences of the leopard (Panthera pardus). J. Zool. 270, 298–313 (2006).
Crossey, B., Chimimba, C., du Plessis, C., Ganswindt, A. & Hall, G. African wild dogs (Lycaon pictus) show differences in diet composition across landscape types in Kruger National Park, South Africa. J. Mammal. 102, 1211–1221 (2021).
Van Klinken, G. J. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 26, 687–695 (1999).
Lueders-Dumont, J. A., Wang, X. T., Jensen, O. P., Sigman, D. M. & Ward, B. B. Nitrogen isotopic analysis of carbonate-bound organic matter in modern and fossil fish otoliths. Geochim. Cosmochim. Acta 224, 200–222 (2018).
Kast, E. R. et al. Nitrogen isotope evidence for expanded ocean suboxia in the early Cenozoic. Science 364, 386–389 (2019).
Auderset, A. et al. Enhanced ocean oxygenation during Cenozoic warm periods. Nature https://doi.org/10.1038/s41586-022-05017-0 (2022).
Roberts, P. et al. Stable carbon, oxygen, and nitrogen, isotope analysis of plants from a South Asian tropical forest: Implications for primatology. Am. J. Primatol. https://doi.org/10.1002/ajp.22656 (2017).
Blumenthal, S. A., Rothman, J. M., Chritz, K. L. & Cerling, T. E. Stable isotopic variation in tropical forest plants for applications in primatology. Am. J. Primatol. 78, 1041–1054 (2016).
Milano, S. et al. Environmental conditions framing the first evidence of modern humans at Tam Pà Ling, Laos: a stable isotope record from terrestrial gastropod carbonates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 511, 352–363 (2018).
Louys, J. & Roberts, P. Environmental drivers of megafauna and hominin extinction in Southeast Asia. Nature 586, 402–406 (2020).
Bourgon, N. et al. Trophic ecology of a Late Pleistocene early modern human from tropical Southeast Asia inferred from zinc isotopes. J. Hum. Evol. 161, 103075 (2021).
McCormack, J. et al. Zinc isotopes from archaeological bones provide reliable trophic level information for marine mammals. Commun. Biol. 4, 683 (2021).
Jaouen, K. et al. Dynamic homeostasis modeling of Zn isotope ratios in the human body. Metallomics 11, 1049–1059 (2019).
Grupe, G. & Turban-Just, S. Amino acid composition of degraded matrix collagen from archaeological human bone. Anthropol. Anz. 3, 213-226 (1998).
McPherron, S. P. et al. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466, 857–860 (2010).
Sahle, Y., El Zaatari, S. & White, T. D. Hominid butchers and biting crocodiles in the African Plio-Pleistocene. Proc. Natl Acad. Sci. USA 114, 13164–13169 (2017).
Ren, H. et al. Foraminiferal isotope evidence of reduced nitrogen fixation in the ice age Atlantic ocean. Science 323, 244–248 (2009).
Sigman, D. M. et al. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal. Chem. 73, 4145–4153 (2001).
Weigand, M. A., Foriel, J., Barnett, B., Oleynik, S. & Sigman, D. M. Updates to instrumentation and protocols for isotopic analysis of nitrate by the denitrifier method. Rapid Commun. Mass Spectrom. 30, 1365–1383 (2016).
Vonhof, H. et al. High‐precision stable isotope analysis of <5 μg CaCO3 samples by continuous‐flow mass spectrometry. Rapid Commun. Mass Spectrom. 34, e8878 (2020).
Hare, V. J., Loftus, E., Jeffrey, A. & Ramsey, C. B. Atmospheric CO2 effect on stable carbon isotope composition of terrestrial fossil archives. Nat. Commun. 9, 1–8 (2018).
Richards, M. P. & Hedges, R. E. M. Stable isotope evidence for similarities in the types of marine foods used by late Mesolithic humans at sites along the Atlantic Coast of Europe. J. Archaeol. Sci. 26, 717–722 (1999).
Hempson, G., Archibald, S. & Bond, W. A continent-wide assessment of the form and intensity of large mammal herbivory in Africa. Science 350, 1056–1061 (2015).
Bothma, Jd. P. Water-use by southern Kalahari leopards. South Afr. J. Wildl. Res. 35, 131–137 (2005).
Wilson, D. E. & Mittermeier, R. A. Handbook of the Mammals of the World (Lynx, 2009).
Hamilton, W. J. Namib Desert chacma baboon (Papio ursinus) use of food and water resources during a food shortage. Madoqua 1986, 397–407 (1986).
Cerling Harris, J. M. & Passey, B. H. Diets of East African bovidae based on stable isotope analysis. J. Mammal. 84, 456–470 (2003).
Acknowledgements
We thank F. Rubach, S. Brömme, M. Schmitt, and B. Hinnenberg (Climate Geochemistry Department, Max Planck Institute for Chemistry, Germany) for technical support, D. Winkler (Zoological Institute, University of Kiel, Germany) and T. Kaiser (Center of Natural History, University of Hamburg, Germany) for assistance in sampling the modern African fauna. We thank J. Broska and P. Held for collagen preparation and δ13C/δ15N analysis of bone collagen. We also thank S. Luangaphay (Department of National Heritage, Ministry of Information and Culture in Vientiane, Laos) for the authorization to study the published fauna of Tam Hay Marklot, P.O. Antoine, who aided in taxonomic identification and analysis of the fauna, and researchers who are part of the Laos project and participated in the fieldwork (F. Demeter, L. Shackelford, P. Duringer, J.L. Ponche, Q. Boesch, E. Patole-Edoumba, T.E. Dunn, A. Zachwieja, E. Suzzoni, S. Frangeul, S. Duangthongchit, T. Sayavonkhamdy, P. Sichanthongtip, and D. Sihanam). This study was funded by the Max Planck Society (A. Martínez-García), the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program (Grant Agreement 681450) (ERC Consolidator Grant Agreement to T. Tütken); the Paul Crutzen Nobel Prize fellowship of the Max Planck Society to N.N. Duprey; and the Deutsche Forschungsgemeinschaft (DFG) Grant LU 2199/1-2 and the Emmy Noether Fellowship LU 2199/2-1 to T. Lüdecke. Prior method development work was supported by the Scott Fund of the Department of Geosciences, Princeton University (to D.M.S.).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Author contributions: J.N.L., T.L., T.T., and A.M.-G. designed the research; J.N.L. and T.L. performed enamel N isotope analyses in the laboratory of A.M.G. with assistance from A.D.F. and N.N.D.; T.T. contributed the bone collagen C and N isotope data; J.N.L. and T.L. performed C and O isotope analyses in the laboratory of H.V., J.N.L. and T.L. analyzed the data; A.-M.B. conducted taxonomic identification and analysis of the fauna; V.S., A.M.B., and N.B. represent the LAOS project team; D.M.S., T.T., and A.M.-G. assisted in the interpretation of the data; J.N.L. and T.L. wrote the paper. All authors contributed to the interpretation of the data and provided input to the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Vincent Balter, Julia Tejada and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Michelle Lawing and Joao Valente. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leichliter, J.N., Lüdecke, T., Foreman, A.D. et al. Tooth enamel nitrogen isotope composition records trophic position: a tool for reconstructing food webs. Commun Biol 6, 373 (2023). https://doi.org/10.1038/s42003-023-04744-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04744-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.