Abstract
Small heat shock proteins (sHSPs) are chaperones with well-characterized roles in heat stress, but potential roles for sHSPs in desiccation tolerance have not been as thoroughly explored. We identified nine sHSPs from the tardigrade Hypsibius exemplaris, each containing a conserved alpha-crystallin domain flanked by disordered regions. Many of these sHSPs are highly expressed. Multiple tardigrade and human sHSPs could improve desiccation tolerance of E. coli, suggesting that the capacity to contribute to desicco-protection is a conserved property of some sHSPs. Purification and subsequent analysis of two tardigrade sHSPs, HSP21 and HSP24.6, revealed that these proteins can oligomerize in vitro. These proteins limited heat-induced aggregation of the model enzyme citrate synthase. Heterologous expression of HSP24.6 improved bacterial heat shock survival, and the protein significantly reduced heat-induced aggregation of soluble bacterial protein. Thus, HSP24.6 likely chaperones against protein aggregation to promote heat tolerance. Furthermore, HSP21 and HSP24.6 limited desiccation-induced aggregation and loss of function of citrate synthase. This suggests a mechanism by which tardigrade sHSPs promote desiccation tolerance, by limiting desiccation-induced protein aggregation, thereby maintaining proteostasis and supporting survival. These results suggest that sHSPs provide a mechanism of general stress resistance that can also be deployed to support survival during anhydrobiosis.
Similar content being viewed by others
Introduction
Desiccation is an extensive stress on cells and organisms1,2. One of the subcellular consequences of drying is protein denaturation and aggregation, which can lead to loss of enzymatic function3,4,5. Molecular shield proteins have been proposed to bind partially unfolded proteins during desiccation to limit contact with other denatured proteins and the formation of toxic aggregates3,6. Late embryogenesis abundant (LEA) proteins from desiccation-tolerant organisms are one family of protectants that have been shown to fulfill this role4,7,8,9,10,11. A limited number of other proteins have also been shown to act as molecular shields, but there are likely many other proteins that carry out a similar function to prevent desiccation-induced aggregation and loss of protein function6,12,13.
Small heat shock proteins (sHSPs) are small (12–43 kDa) proteins that contain an alpha-crystallin domain flanked by disordered N- and C-terminal sequences14,15. The regions flanking the alpha-crystallin domain often contribute to oligomerization of the protein—a feature that is conserved amongst many sHSPs and can be important for their function16,17,18,19. sHSPs act as chaperones to limit protein aggregation at high temperatures, and sHSP mutations have been associated with several human diseases20,21,22,23,24,25. Accordingly, many of these proteins are upregulated during stress26,27. In contrast to other heat shock proteins like HSP70, sHSPs are not ATP-dependent, instead functioning as “holdases” to limit protein aggregation until unfolded clients can either be refolded or degraded—a function akin to that of proposed molecular shield proteins during desiccation28. Plant sHSPs have been shown to improve osmotic stress survival and contribute to drought tolerance29,30,31,32. However, a role for sHSPs in animal desiccation tolerance has been less well-explored.
sHSPs have been reported to be highly abundant and upregulated during desiccation in multiple anhydrobiotic animals33,34,35,36,37. A small heat shock protein, p26, from the brine shrimp Artemia is very highly expressed in embryonic cysts that are destined for diapause38. p26 constitutes approximately 15% of the total non-yolk protein in these cysts, which are resistant to multiple stresses including anoxia, heat, and desiccation33. p26 improves thermotolerance of brine shrimp and heterologous expression can increase heat shock survival of bacteria39. In vitro, p26 could protect the enzyme citrate synthase from thermal inactivation40. Further, when combined with trehalose, p26 could improve viability of dried human cells41. In the tardigrade Milnesium tardigradum, a sHSP was highly abundant in both active and anhydrobiotic animals42. In a study of the transcriptional response to desiccation in C. elegans, the most upregulated gene during desiccation, with over 700-fold induction, encodes for a small heat shock protein37. Additionally, two C. elegans sHSP mutants had reduced survival when desiccated37. To our knowledge, sHSPs have not been tested for a role in desiccation tolerance in vitro in the absence of trehalose, nor using tardigrade-derived sHSPs. We hypothesized that sHSPs may function to chaperone against desiccation-induced protein aggregation, acting as molecular shields to support desiccation tolerance.
Tardigrades are able to withstand extreme stresses including desiccation, yet the molecular mechanisms they employ to survive are only beginning to be understood2,43,44,45,46,47,48,49,50. We identified a family of sHSPs from the tardigrade Hypsibius exemplaris to specifically test the hypothesis that sHSPs can function as molecular shields and limit desiccation-induced protein aggregation. We analyzed published RNA-seq studies and saw that tardigrade sHSPs were highly expressed and in some cases upregulated by desiccation48,49. We found that multiple sHSPs, when expressed heterologously in bacteria, were sufficient to improve desiccation survival. We purified HSP21 and HSP24.6 and found that they formed large, polydisperse oligomeric complexes, similar to what has been reported for other members of the sHSP family in plants and mammals14. We demonstrated that HSP21 and HSP24.6 could function as chaperones to limit heat-induced aggregation of the model enzyme citrate synthase (CS) and aggregation of total soluble bacterial protein lysate. Further, these sHSPs limited desiccation-induced aggregation and loss of function of CS, suggesting a role as a molecular shield in protecting proteins during desiccation.
Results
A family of sHSPs in H. exemplaris
We identified nine small heat shock proteins from the most recent genome annotation (v3.1.5) of the tardigrade H. exemplaris (Fig. 1a)49. We named each of the proteins by its predicted molecular weight (Supplementary Table 1). The protein sequences of the nine sHSPs we identified are consistent with conserved features of other sHSPs, i.e., with an alpha-crystallin domain (ACD) flanked by an N-terminal region and short C-terminal region15. Interestingly, three of the sHSP genes, HSP17, HSP19, and HSP20, are located next to each other on the same genomic scaffold (Fig. S1). The DNA coding sequences of these three genes share over 90% sequence identity, suggesting that these three genes may have arisen from gene duplication events, which has been suggested as a general mode of sHSP evolution51,52,53,54.
a Nine sHSPs were identified that conform to the typical structure containing an alpha-crystallin domain flanked by disordered N-terminal and C-terminal domains. b Seven of nine sHSPs were upregulated during desiccation in RNA-seq data from Boothby et al. 2017 (HSP17, HSP19, HSP20, HSP21, HSP23, HSP24.6, and HSP38, FDR < 0.01). sHSP transcripts are highlighted on a plot of expression fold-change compared to relative abundance from mRNA-seq. c Only one sHSP (HSP23) was significantly upregulated during desiccation (FDR < 0.01) in RNA-seq data from Yoshida et al. 2017.
sHSPs are highly expressed in tardigrades
Two independent studies have conducted RNA-seq experiments to probe transcriptional changes in H. exemplaris during desiccation48,49. Here, we re-analyzed these data, mapping reads from both studies to a single version of the genome—the same version from which we identified the sHSPs49. In the Boothby et al. (2017) dataset, seven of the nine sHSPs were significantly upregulated in drying tardigrades at an FDR < 0.01 (Fig. 1b). In the Yoshida et al. (2017) dataset, only HSP23 was significantly upregulated in dried tardigrades (Fig. 1c). These differences might be explained by stage of drying (i.e., drying vs. dried), desiccation conditions, or sample collection methods used in these two studies. Regardless of the relevant differences, in each study, transcripts encoding several of the sHSPs were among the most highly abundant transcripts. We conclude that the transcripts encoding these sHSPs are highly abundant as animals experience desiccation.
Several sHSPs can improve bacterial desiccation tolerance
While abundance of sHSPs in desiccation tolerant organisms like nematodes, brine shrimp, and now tardigrades suggests that sHSPs may help proteins resist desiccation, we were motivated to test this hypothesis directly. To first determine if tardigrade sHSPs can promote desiccation tolerance, we heterologously expressed each sHSP in BL21 E. coli. Each construct was well-expressed in cells (Fig. S2a). However, HSP23 and HSP25, along with a truncated GFP control, had limited solubility in bacteria (Fig. S2b). We desiccated bacteria overexpressing each sHSP. Expression of HSP21, HSP24.6, HSP25.1, and HSP38 significantly improved desiccation survival relative to GFP-expressing control bacteria (Fig. 2). Bacteria expressing each of these four proteins exhibited over 100-fold improvement in desiccation survival relative to GFP-expressing control bacteria. Thus, at least some sHSPs are sufficient to improve desiccation tolerance. The limited solubility of HSP23 and HSP25 likely limit their ability to protect bacteria during desiccation, so it remains unclear if they would be protective if soluble. Other sHSPs like HSP17, HSP19, and HSP20 did not improve bacterial survival even though they were soluble, suggesting that they lack some property that is shared amongst at least HSP21, HSP23, HSP25.1, and HSP38.
Desiccation survival of BL21 E. coli was significantly different across conditions (p < 0.001, 1-way ANOVA, n = 3–6). Bacterial survival was significantly improved relative to GFP-expressing bacteria by heterologous expression of HSP21 (p < 0.001), HSP24.6 (p < 0.001), HSP25.1 (p < 0.001), or HSP38 (p < 0.001). Dunnett’s post-hoc test was used to compare each condition to GFP-expressing controls. Individual data points represent independent replicates and lines depict mean survival. ***p < 0.001.
HSP21 and HSP24.6 can form higher-order complexes
We were curious about the biochemical properties of sHSPs that could allow them to promote desiccation tolerance. HSP21 and HSP24.6 were the two proteins that conferred the largest improvement of bacterial desiccation survival (Fig. 2). Intriguingly, these were also the two genes that were most upregulated by desiccation in the Boothby et al. 2017 dataset (Fig. 1b). Therefore, we prioritized these two proteins for further characterization. To purify recombinant HSP21 and HSP24.6 for in vitro biochemistry, we added a 6xHis::TEV tag to each protein, purified the proteins with NiNTA columns, and cleaved the His tags with TEV protease (Fig. S2c, d).
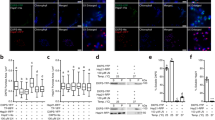
Many sHSPs from different species are reported to form oligomeric complexes17,55,56,57,58. For example, p26 of Artemia forms a roughly 700 kDa multimeric assembly of ~27 monomers40. To determine if HSP21 and HSP24.6 form similar oligomeric assemblies, we visualized protein by negative staining TEM and determined the size distribution of protein complexes by mass photometry. Negative staining TEM of HSP21 revealed the presence of large structures that had some variability in size and shape (Fig. 3a). Mass photometry analysis showed a population of protein at low molecular weight that could represent monomers or small oligomeric complexes, and a second population of protein spanning a wide range of masses (Fig. 3b). This latter, broad peak likely represents the complexes seen with TEM. In contrast to HSP21, HSP24.6 formed slightly more homogenously-sized structures approximately 15–20 nm in diameter (Fig. 3c). By mass photometry we again detected peaks indicative of two populations of mass species. In this case, the peak representing larger multimeric complexes was more pronounced. Given the breadth of the peaks and range of masses included, we consider it likely that oligomeric complexes of variable numbers of monomers are represented. Assembly of higher-order complexes does not appear to be sensitive to particular buffers. Visualization of protein diluted in water (Fig. 3a, c), yielded similar results to protein diluted in TEN buffer (Fig. S3). Formation of HSP21 and HSP24.6 multimeric assemblies is consistent with reports from other sHSPs14,59, and suggests that they may therefore harbor similar biochemical functions as chaperones.
a Negative staining TEM of HSP21 reveals large assemblies of variable size and shape. b Mass photometry analysis indicates two populations of HSP21, a peak of smaller mass species—likely comprising limited oligomeric assembly—and a broad peak of larger mass species that likely includes a range of oligomeric states. c Negative staining TEM of HSP24.6 shows particles of more regular size and shape than HSP21. d Mass photometry analysis reveals HSP24.6 protein complexes of larger size that likely represent large oligomeric assemblies, in addition to a peak at lower mass representing smaller assemblies. Proteins were diluted in 1x PBS for mass photometry, and in molecular grade water for TEM.
sHSPs can improve bacterial heat shock survival and chaperone against heat-induced protein aggregation
The canonical chaperone function of sHSPs is to limit heat-induced protein aggregation. Citrate synthase (CS) is a common model enzyme used for studies of protein aggregation3,60,61. We tested if HSP21 or HSP24.6 could limit temperature-induced aggregation of CS. Heating at 43 °C induced aggregation of the enzyme that could be reduced by the addition of either HSP21 or HSP24.6 (Fig. 4a). HSP24.6 supplementation resulted in a more significant reduction of protein aggregation. In contrast, the addition of bovine serum albumin (BSA), used as a control, at the same molar ratio did not reduce CS aggregation. At higher concentrations, each sHSP could further limit thermal aggregation of CS, although BSA also had an effect at this concentration and was indistinguishable from HSP21 (Fig. S4). These data indicate that HSP24.6 is more effective at chaperoning against heat-induced aggregation.
a HSP21 and HSP24.6 limit heat-induced aggregation of citrate synthase at 43 °C. Aggregation is plotted as the change in A340 relative to that of 5 µM citrate synthase alone at 2 h. Datapoints from four biological replicates are shown along with a fitted line and 95% confidence interval. b) Heterologous expression of HSP24.6 improved heat shock survival of BL21 E. coli (p = 0.02, Dunnett’s test, n = 4). c HSP21 and HSP24.6 limited heat-induced aggregation of the water soluble BL21 E. coli proteome. In vitro aggregation of soluble protein following 1 h at 52 °C was significantly different across conditions shown (p < 0.001, 1-way ANOVA, n = 3–4). HSP21 (p = 0.01, Dunnett’s test, n = 4) and HSP24.6 (p < 0.001, Dunnett’s test, n = 4) reduced aggregation, while BSA (p = 0.96, n = 3, Dunnett’s test) did not have an effect. sHSPs or BSA were added at a 1:1 mass ratio with E. coli protein. d) Soluble (supernatant, S) and insoluble (pellet, P) protein fractions were assessed by SDS-PAGE. A representative Coomassie-stained gel shows soluble and insoluble protein following heat shock at 52 °C for 1 h. The pelleted insoluble fraction was concentrated 4× before loading. *p < 0.05, ***p < 0.001.
To test if HSP21 and HSP24.6 could improve bacterial heat shock survival, we exposed BL21 E. coli overexpressing these proteins to heat stress. We chose a stress of 52 °C for 1 h as a condition for heat stress based on the survival of GFP-expressing bacteria across a range of temperatures (Fig. S5a). Heterologous expression of HSP24.6, but not HSP21, improved bacterial heat shock survival (Fig. 4b); perhaps surprisingly, none of the other tardigrade sHSPs significantly improved bacterial heat shock survival (Fig. S5b).
We hypothesized that HSP24.6 improved bacterial heat shock survival by chaperoning against protein aggregation. To test this hypothesis, we exposed total soluble protein lysate from the same strain of bacteria to heat stress. Indeed, we observed significant aggregation of the bacterial proteome when measuring light scattering (A340) or by separating soluble (supernatant, S) and insoluble (pellet, P) fractions by centrifugation and visualizing these fractions with SDS-PAGE (Fig. 4c, d). Supplementation with a 1:1 mass ratio of HSP21 led to a modest reduction in protein aggregation, and the addition of HSP24.6 markedly reduced protein aggregation (Fig. 4c, d). We did not observe a significant effect of BSA in limiting protein aggregation. These results suggest that HSP21 has modest chaperone activity to limit thermal aggregation of proteins and that HSP24.6 is highly effective at chaperoning against protein aggregation at high temperature. Total soluble protein lysate from bacteria also contains a wide variety of proteins with unique biophysical properties. SDS-PAGE analysis of soluble and insoluble protein shows that HSP21 and HSP24.6 can promote the solubility of a broad range of proteins (Fig. 4d), indicating that the chaperone function of these sHSPs is likely general rather than specific to particular clients.
HSP21 and HSP24.6 can limit desiccation-induced protein aggregation
We were specifically interested in the possibility that tardigrade sHSPs may be able to function in the context of desiccation by acting as molecular shields. We tested the ability of HSP21 and HSP24.6 to limit desiccation-induced aggregation of citrate synthase. We purified a third tardigrade sHSP, HSP17 (Fig. S2e), that was not sufficient to improve bacterial desiccation tolerance (Fig. 2), with the expectation that this protein may serve as a negative control. We also included lysozyme as a negative control that we did not expect to impact the aggregation of CS. BSA was included as a positive control with known potency as an excipient during desiccation62,63. As further evidence for a protective role of BSA, we found that it was sufficient to improve bacterial desiccation survival, despite low levels of expression (Fig. S6). Multiple rounds of desiccation led to increasing aggregation of CS, and a range of concentrations of sHSPs and BSA, could alter the extent of aggregation (Fig. 5a–e)3. None of the experimental protectant proteins when desiccated alone demonstrated significant aggregation as measured by light scattering (A340) (Fig. S7). The addition of HSP21 reduced the aggregation of CS after one round of desiccation and rehydration (p = 0.002, 1-way ANOVA, n = 4–9). HSP24.6 significantly reduced aggregation of CS after both one (p < 0.001, 1-way ANOVA, n = 4–9) and two rounds of desiccation (p = 0.003, 1-way ANOVA, n = 4–9, Fig. 5f). Addition of BSA also protected CS from desiccation-induced aggregation (p < 0.001, 1-way ANOVA, n = 4–9). In contrast, supplementation with HSP17 caused an increase in aggregation (p = 0.03, 1-way ANOVA, n = 3–9). Interestingly, there were minimal differences in CS solubility across experimental conditions after multiple rounds of desiccation (Fig. S8). It is possible that some additives change the size or density of aggregates without shifting the overall balance of CS solubility. For example, the formation of smaller aggregates in the presence of some protectants could result in less light scattering while not impacting the solubility of total protein.
Light scattering (A340) values indicate aggregation of 5 µM citrate synthase over two rounds of desiccation and rehydration and the effects of adding HSP17 (a), HSP21 (b), HSP24.6 (c), BSA (d), or lysozyme (e). f After two rounds of desiccation and rehydration, the change in A340 across a range of concentration of additives showed an effect of concentration on aggregation for HSP17 (p = 0.03, 1-way ANOVA, n = 3–9), HSP24.6 (p = 0.003, 1-way ANOVA, n = 4–9), and BSA (p < 0.001, 1-way ANOVA, n = 4–9). g Citrate synthase was protected from desiccation-induced loss of function when supplemented with 10 µM HSP17 (p = 0.009), 5 µM HSP21 (p < 0.001),10 µM HSP21 (p < 0.001), 1 µM HSP24.6 (p = 0.05), 10 µM HSP24.6 (p < 0.001), 1 µM BSA (p = 0.006), 5 µM BSA (p < 0.001), or 10 µM BSA (p < 0.001). Enzyme activity is plotted as a percentage of non-desiccated citrate synthase. P-values resulted from Dunnett’s tests comparing to desiccated CS alone, n = 5–10. Mean values and standard deviation are plotted in (a–g). *p < 0.05, **p < 0.01, ***p < 0.001.
Ultimately, protein misfolding and aggregation can result in loss of protein function. To more directly test the protective ability of sHSPs during desiccation, we measured CS activity after four rounds of desiccation and rehydration. At a 2:1 molar ratio, HSP17, HSP21, and HSP24.6 each prevented the desiccation-induced loss of CS activity (Fig. 5g). Lower concentrations of HSP21 (1:1 molar ratio) and HSP24.6 (1:5 molar ratio) were sufficient to limit the loss of CS activity. At a concentration of 10 µM HSP21 and HSP24.6 functioned similarly to BSA (despite, with a molecular weight of 66 kDa, an equimolar amount of BSA representing approximately 3× the mass of sHSP monomers). It is interesting that HSP17 could limit the loss of CS activity despite an apparent increase in aggregation after two rounds of desiccation. Several sHSPs from yeast, C. elegans, and E. coli have been shown to exert cytoprotective functions by promoting the sequestration of misfolded proteins in inclusions64. It is possible that HSP17 acts similarly to sequester CS and protect it from irreversible denaturation. Overall, we conclude that HSP21 and HSP24.6 can limit desiccation-induced aggregation of CS and can allow for the retention of enzymatic function.
Discussion
Small heat shock proteins are well-studied chaperones that are upregulated upon several stress conditions, and mutations of sHSPs has been associated with human disease25,65. We were initially intrigued by reports of the high abundance of sHSPs in some desiccation-tolerant organisms like Artemia and C. elegans33,34,37. We found that in two independent RNA-seq datasets of H. exemplaris, sHSPs were among the most abundant transcripts detected (Fig. 1b, c). The Boothby et al. dataset showed significant induction of many sHSPs while the Yoshida et al. dataset did not. Another study assessed expression of two sHSPs from the tardigrade Milnesium tardigradum during heat shock and anhydrobiosis, with one sHSP being significantly upregulated by thermal stress, but neither by anhydrobiosis66. It is possible that sHSPs are more transcriptionally responsive to temperature fluctuations, which could explain differences in induction between the two datasets if there were differences in temperature when tardigrades were collected. It is also likely that sHSPs are variably expressed during different stages of drying and particularly sensitive to the time of sampling during the process of tun formation and preparation for anhydrobiosis. Besides differences in induction, RNA-seq data show the relative abundance of sHSP transcripts even in unstressed tardigrades. Transcriptional induction may indicate an adaptive response, but is not a necessary condition for function if proteins are constitutively present. Therefore, this result suggests that perhaps these proteins are constitutively present and can stabilize the proteome under normal conditions in addition to during stresses like heat or desiccation, similar to what has been proposed for sHSPs in other organisms, including C. elegans and mammals, where selected sHSPs have been shown to participate in development and differentiation67,68.
Although tardigrades are renowned for their desiccation survival, they are not noted for their survival of heat stress unless in the tun state69,70. This raises the possibility that some tardigrade sHSPs may have evolved properties that enable them to function during desiccation, perhaps at the expense of thermal tolerance. Given the typical association with sHSPs and heat stress, we were somewhat surprised to find that HSP21, HSP24.6, HSP25.1, and HSP38 could each improve bacterial desiccation survival (Fig. 2), but only HSP24.6 was sufficient to improve bacterial heat shock survival (Fig. S5b). Notably, not all sHSPs function during heat shock, and some act as chaperones instead during cold stress or other conditions71. It is possible that some tardigrade sHSPs have, in fact, undergone selection for function during desiccation. However, using bacteria as a system for heterologous expression makes these differences challenging to interpret. A clear comparison of sHSP function during stresses is confounded by variables like the levels of protein expression or solubility, conditions for bacterial heat stress and desiccation that may not be optimal for the function of tardigrade proteins, or other caveats of putting eukaryotic proteins in prokaryotic cells. Nonetheless, heterologous expression experiments like these are a powerful and efficient way to test for protectants that are sufficient to promote survival and generalizable beyond the specific biology of a tardigrade. With an eye towards developing better protectants to stabilize biomaterials or produce drought-tolerant crops, these are the types of protectants that are of greatest interest. If tardigrade proteins are uniquely suited as desicco-protectants, it is intriguing to consider the possibility for such applications.
To gain some insight into whether sHSPs from tardigrades may be uniquely optimized for conditions of desiccation, we tested if human sHSPs could provide similar improvement to bacterial desiccation survival. Heterologous expression of five of the ten human sHSPs (HSPB1/Hsp27, HSPB4/alpha A crystallin, HSPB5/alpha B crystallin, HSPB7/cvHSP, and HSPB10/ODF1) improved bacterial desiccation tolerance (Fig. S9). Again, high expression levels and solubility may explain some of the ability of particular sHSPs like HSPB1, HSPB4, and HSPB5 to improve bacterial desiccation survival (Fig. S9b, c). These results suggest that tardigrade sHSPs may not harbor particularly unique chaperone properties for desiccation, but rather, that animal sHSPs in general might harbor potency to contribute to desiccation tolerance. Thus, desicco-protection is emerging as a conserved property of some sHSPs across different species. So why are human cells still desiccation-sensitive if some human sHSPs can also promote bacterial desiccation tolerance? Desiccation is a harsh stress that causes widespread cellular damage (for example to DNA, RNA, and membranes) that precludes survival even if stress to the proteome is lessened. Human sHSPs may limit protein aggregation and improve survival in other, less severe, contexts like osmotic stress that mimic the depletion of subcellular water and the concentration of cytosolic components. It is further possible that in human cells sHSPs may not be present in appropriate quantities or subcellular locations required for function, or that other protectants must also be provided. For example, heterologous expression of the sHSP p26 from brine shrimp did not improve the viability of drying 293H cells unless trehalose was also present41.
We found that HSP21 and HSP24.6 form oligomeric complexes (Fig. 3), similar to what has been described for other sHSPs18,40,55,56,57,58. In some cases, the ability of sHSPs to oligomerize has been shown to be essential for their chaperone properties, and some of the human sHSPs that could protect bacteria from desiccation are also known to oligomerize72,73,74,75. We speculate that oligomerization of HSP21 and HSP24.6 may be required for their activity in limiting heat- and desiccation-induced protein aggregation. However, further work is required to test this hypothesis directly. The dynamic equilibrium of assembly and disassembly of sHSP oligomeric complexes is often sensitive to environmental factors like temperature or pH19, which raises the possibility that these, or other factors like osmolyte concentration, could alter the assembly of sHSP complexes during drying. It is also possible that these sHSPs may hetero-oligomerize in the presence of other sHSPs to form alternative functional assemblies76,77. Future analysis of native sHSP assemblies may inform the extent to which such interactions exist and are important in the context of desiccation.
In conclusion, we define a family of sHSPs from the tardigrade H. exemplaris and present evidence for their involvement in the desiccation response. Many sHSP transcripts were present in significant quantities in tardigrades, and overexpression of several of these proteins could improve bacterial desiccation survival. HSP24.6 was particularly effective at promoting desiccation survival, likely by limiting protein aggregation. HSP24.6 also improved bacterial heat shock survival and limited heat-induced protein aggregation. Thus, HSP24.6 is a general chaperone that may function to maintain proteostasis in varying stress conditions. It is possible that sHSPs may have other functions beyond limiting protein aggregation that could contribute to desiccation survival. sHSPs have been shown in some contexts to impact diverse cellular components and processes such as protein degradation, membrane fluidity, cytoskeletal organization, and apoptosis24,78,79,80,81,82,83. sHSPs contain intrinsically disordered regions and can undergo liquid-liquid phase separation, forming or being recruited inside biomolecular condensates68,84,85,86,87. Biomolecular condensates are typically enriched for aggregation-prone disordered proteins, and mammalian sHSPs were shown to prevent condensate conversion from a dynamic liquid-like state into an irreversibly aggregated state, with important implications for cellular stress responses and human disease85,86,87. Whether tardigrade sHSPs might also help to maintain reversible phase-transitions and whether this may contribute to desiccation survival, similar to what has been suggested for LEA proteins88, is still unknown. It will be of further interest to determine the properties that allow some sHSPs to function during desiccation and to explore the endogenous roles of sHSPs in tardigrades.
Methods
Identification and cloning of H. exemplaris sHSPs
Small heat shock proteins of Hypsibius exemplaris were identified by BLAST using the ten human HSPB sequences and 12 Drosophila sHSP sequences as BLAST queries against the H. exemplaris transcriptome version 3.1.549. Protein sequences from top hits were surveyed for alpha crystallin domains and regions of disorder, characteristics of conserved sHSPs. These features were annotated based on identification of the alpha crystallin domain from NCBI conserved domain searches and regions of predicted disorder annotated by prDOS89,90. Protein sequences of sHSPs are included in Supplementary Table 2.
sHSPs were cloned into pDest17 for bacterial expression. Tardigrade (Hypsibius exemplaris strain Z151) RNA was isolated by established methods and converted to cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen, 18080051)91. Primers were designed to amplify transcripts from total cDNA, and coding sequences were amplified and assembled into linearized pDest17 using NEBuilder Hifi Assembly 2× master mix (NEB, E2621). NEB 5-alpha E. coli were transformed with the assembly product, and individual colonies were grown overnight and miniprepped. The gene insert regions were sequenced to confirm that the coding region matched that of the genome (3.1.5) and were correctly assembled into the vector. Synonymous mutations in coding sequences were present in some cases. BV898_14401 (HSP20) was unable to be cloned from cDNA so was instead cloned into the expression vector from a synthesized gBlock (Integrated DNA Technologies).
Analysis of desiccation-induced expression
Two previous studies conducted RNA-seq on hydrated and desiccated tardigrades48,49. Here, we have re-analyzed those datasets by mapping reads to the most recent version of the genome (v3.1.5). Reads were downloaded from the NCBI SRA database (SRP098585: GSM2472501 through GSM2472506 and PRJNA: SRX2663153, SRX2663154, SRX2527798, SRX2661843, SRX2661844, SRX2527616). Reads were mapped using Bowtie2 and counts were assigned with Featurecounts and using the annotation file associated with the genome version 3.1.5. Reads were aggregated at the level of genes. Only genes with more than one count in at least two samples were kept for differential expression analysis. Transcript abundance, fold changes, and FDR values were determined with EdgeR92.
Bacterial desiccation and heat shock survival
Individual sHSPs were expressed in E. coli BL21 AI (Invitrogen, C607003) to determine if heterologous expression could confer desiccation tolerance. BL21 AI cells were transformed with the sequence-verified expression plasmids. Bacteria were grown overnight in 5 mL LB with Ampicillin and diluted 1:20 into LB with Ampicillin and 0.2% L-arabinose to induce expression. Induction cultures were grown for 4 h at 37 °C with shaking. OD600 of induced bacteria was measured and the densities of bacteria were normalized. A dilution series of bacteria was plated to determine the control cfu. Bacteria were pelleted, supernatant removed, and placed in a speedvac for overnight desiccation. Bacteria were then rehydrated with the same volume in which they were initially suspended and a dilution series was plated. Survival was calculated as the cfu after desiccation divided by the control cfu. For heat shock experiments, bacteria were prepared as for desiccation and heated for 1 h in a Thermocycler. Non-heated samples were kept as controls. Serial dilutions were plated and survival calculated as the ratio of cfu from heated samples to controls.
Protein purification
To purify HSP17, HSP21, and HSP24.6, a 6× His epitope tag and TEV cleavage site were cloned onto the N-terminus of the coding sequence of each gene in expression plasmids. Expression vectors were sequence-verified and transformed into BL21 AI E. coli (Invitrogen, C607003). Overnight cultures of bacteria in LB with Ampicillin were inoculated at a 1:20 ratio into 1–2 L of LB with Ampicillin and 0.2% L-arabinose for induction. Cultures were induced for 6 h at 37 °C with shaking. Bacteria were harvested by centrifugation at 5000 rpm for ten minutes and concentrated into pellets.
Bacterial pellets were resuspended in NiNTA binding/wash buffer A (20 mM sodium phosphate buffer, 500 mM NaCl, 20 mM imidazole, pH 7.4) with lysozyme (Sigma, L6876), DNAse I (Thermo, 18047019), and a protease inhibitor cocktail (Fisher, PIA32965) and sonicated on ice. Lysates were spun at 14,000 × g for 1 h to clear cellular debris and insoluble protein. Soluble and insoluble fractions of cell lysate were run on gels to ensure that protein to be purified was present in the soluble fractions. Lysates were filtered (0.45 µm filter) and loaded onto a HisTrap column (Cytiva) with 1 mL binding capacity. The column was washed with NiNTA wash buffer (with 20 mM imidazole) and eluted with the same buffer with 250 mM imidazole. The column was then flushed with buffer with 500 mM imidazole.
Protein eluted from the column was treated with TEV protease to cleave the 6× His tag. The protein was dialyzed into HBS for the TEV digest. TEV protease was added at a mass ratio of 25:1 (target protein:protease) and allowed to proceed overnight at 4 °C. The digest was dialyzed back into NiNTA buffer A and passed back over the regenerated, re-equilibrated column. The flowthrough was collected as released target. Uncleaved protein was then eluted with 250 mM imidazole. The cleaved target protein was dialyzed into PBS, aliquoted, and flash frozen in liquid nitrogen. Aliquots were stored at −80 °C.
Negative staining TEM
Protein samples were visualized by negative-stain transmission electron microscopy. Concentrations were 100 μg/mL of protein for samples diluted in water and 50 μg/mL of protein diluted in TEN buffer. A glow-discharged formvar/carbon-coated 400 mesh copper grid (Ted Pella, Inc., Redding, CA) was floated on a 20 µl droplet of the sample suspension for 10 min, transferred quickly to 2 drops of deionized water followed by a droplet of 2% aqueous uranyl acetate stain for 1 min. The grid was blotted with filter paper and air-dried. Samples were observed using a JEOL JEM1230 transmission electron microscope operating at 80 kV (JEOL USA INC., Peabody, MA) and images were taken using a Gatan Orius SC1000 CCD camera with Gatan Microscopy Suite version 3.10.1002.0 software (Gatan, Inc., Pleasanton, CA).
Mass photometry
A Refeyn One mass photometer was used to assess the distribution of mass species of purified sHSPs93,94. Coverslips were prepared by sonication in isopropanol for five minutes, washed in water twice, sonicated for 5 minutes in water, and washed once more in water. Coverslips were dried before applying a sample well cassette. 10 µL of PBS was added to a well and the focus was adjusted. 10 µL of diluted sHSPs were added to each well for final concentrations of 500 nM (HSP21), or 1 µM (HSP24.6). Testing a range of concentrations provided similar results to those reported. The raw contrast values were converted to mass values by normalizing to a mass calibration with NativeMark unstained protein ladder (Thermo, LC0725).
Chaperone assays
To assess the chaperone activity of sHSPs, the aggregation-prone model enzyme citrate synthase was used. 5 µM citrate synthase (Sigma, C3260) was incubated at 43 °C in a BioTek Synergy H1 plate reader with orbital shaking. sHSPs or bovine serum albumin (BSA) were added at a concentration of 1 µM or 5 µM. Absorbance at 340 nm was read at 3 min intervals. To test for aggregation of total E. coli soluble protein, soluble lysate was collected from BL21 AI cells transformed with pUC19. Cells were resuspended in water and sonicated on ice. The crude lysate was spun twice at 14,000 rpm for ten minutes at 4 °C, and the soluble supernatant was retained. Protein was quantified with the BioRad Protein Assay kit. Lysates were set up with 50 µg of the soluble lysate and 50 µg of either sHSP protein or BSA. Protein mixtures were heated at 52 °C for 1 h. The absorbance at 340 nm was read before and after heating. To visualize soluble and insoluble protein, heated samples were centrifuged at 14,000 rpm for ten minutes at 4 °C. The soluble fraction (100 µL) was moved to a new tube. Pelleted insoluble debris was resuspended in 50 µL of sample buffer. These fractions were run on a 4–12% BT gel with 10 µL of soluble protein added to 10 µL of 2× sample buffer and 20 µL of insoluble protein in sample buffer. This is effectively a 4× concentration of the insoluble fraction.
To test for desiccation-induced protein aggregation, 5 µM citrate synthase solutions were prepared in 0.1× PBS and supplemented with varying concentrations of HSP17, HSP21, HSP24.6, BSA, or lysozyme. Solutions were subjected to multiple 2 hr rounds of desiccation in a savant speedvac concentrator and rehydrated in molecular grade water. Light scattering (A340) was measured before desiccation and after successive rounds of desiccation and rehydration.
To determine solubility of CS after two and four rounds of desiccation, rehydrated protein was spun at 14,000 rpm for ten minutes at 4 °C. Soluble supernatant was moved to a new tube and the pelleted insoluble fraction was resuspended in 50 µL of sample buffer and 50 µL of molecular grade water. Soluble and insoluble fractions were run on a 4–12% BT gel with 8 µL of soluble protein added to 8 µL of 2× sample buffer and 16 µL of insoluble protein in sample buffer.
Citrate synthase enzyme activity
A Citrate Synthase Activity Assay Kit from Sigma-Aldrich (MAK193) was used to measure the enzyme activity of citrate synthase before and after desiccation. Because significant enzyme function was retained with two rounds of desiccation, we used four rounds of desiccation as the assay endpoint. Samples were diluted 1:100 to monitor activity. Absorbance at 512 nm was read every five minutes for one hr in a BioTek Synergy H1 plate reader. Activity was calculated according to the kit’s instructions and normalized to the activity of CS from samples that were not desiccated.
Statistics and reproducibility
For gene expression analysis, statistics were calculated by EdgeR and transcripts were assigned an FDR value. For heterologous expression survival experiments in bacteria, a 1-way ANOVA was used to first test for any difference across conditions. If this was significant, a post hoc Dunnett’s test was used to determine which conditions were significantly different from the GFP-expressing control strain. Similarly, for protein aggregation and enzyme activity experiments, 1-way ANOVAs followed by post hoc Dunnett’s tests were employed to determine significant differences from a control. The number of independent biological replicates for each experiment is noted in Figure legends or the text.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Keilin, D. The problem of anabiosis or latent life: history and current concept. Proc. R. Soc. B Biol. Sci. 150, 149–191 (1959).
Hibshman, J. D., Clegg, J. S. & Goldstein, B. Mechanisms of desiccation tolerance: themes and variations in brine shrimp, roundworms, and tardigrades. Front. Physiol. 11, 1–19 (2020).
Goyal, K., Walton, L. J. & Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 388, 151–157 (2005).
Chakrabortee, S. et al. Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proc. Natl Acad. Sci. USA 104, 18073–18078 (2007).
Carpenter, J. F. & Crowe, J. H. Modes of stabilization of a protein by organic solutes during desiccation. Cryobiology 25, 459–470 (1988).
Chakrabortee, S. et al. Intrinsically disordered proteins as molecular shields. Mol. Biosyst. 8, 210–219 (2012).
Furuki, T. et al. Effects of Group 3 LEA protein model peptides on desiccation-induced protein aggregation. Biochim. Biophys. Acta - Proteins Proteom. 1824, 891–897 (2012).
LeBlanc, B. M. & Hand, S. C. Target enzymes are stabilized by AfrLEA6 and a gain of α-helix coincides with protection by a group 3 LEA protein during incremental drying. Biochim. Biophys. Acta - Proteins Proteom. 1869, 140642 (2021).
Hatanaka, R. et al. An abundant LEA protein in the anhydrobiotic midge, PvLEA4, acts as a molecular shield by limiting growth of aggregating protein particles. Insect Biochem. Mol. Biol. 43, 1055–1067 (2013).
Liu, Y., Chakrabortee, S., Li, R., Zheng, Y. & Tunnacliffe, A. Both plant and animal LEA proteins act as kinetic stabilisers of polyglutamine-dependent protein aggregation. FEBS Lett. 585, 630–634 (2011).
Hibshman, J. D. & Goldstein, B. LEA motifs promote desiccation tolerance in vivo. BMC Biol. 19, 1–22 (2021).
Chakrabortee, S. et al. Catalytic and chaperone-like functions in an intrinsically disordered protein associated with desiccation tolerance. Proc. Natl Acad. Sci. USA 107, 16084–16089 (2010).
Kim, S. X., Çamdere, G., Hu, X., Koshland, D. & Tapia, H. Synergy between the small intrinsically disordered protein Hsp12 and trehalose sustain viability after severe desiccation. Elife 7, 1–20 (2018).
Haslbeck, M. & Vierling, E. A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. J. Mol. Biol. 427, 1537–1548 (2015).
Haslbeck, M., Weinkauf, S. & Buchner, J. Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 294, 2121–2132 (2019).
Laksanalamai, P. & Robb, F. T. Small heat shock proteins from extremophiles: a review. Extremophiles 8, 1–11 (2004).
Giese, K. C. & Vierling, E. Changes in oligomerization are essential for the chaperone activity of a small heat shock protein in vivo and in vitro. J. Biol. Chem. 277, 46310–46318 (2002).
Moutaoufik, M. T., Morrow, G., Finet, S. & Tanguay, R. M. Effect of N-terminal region of nuclear Drosophila melanogaster small heat shock protein DmHsp27 on function and quaternary structure. PLoS One 12, 1–19 (2017).
Janowska, M. K., Baughman, H. E. R., Woods, C. N. & Klevit, R. E. Mechanisms of small heat shock proteins. Cold Spring Harb. Perspect. Biol. 11, a034025 (2019).
Jakob, U., Gaestel, M., Engel, K. & Buchner, J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 268, 1517–1520 (1993).
Horwitz, J. α-Crystallin can function as a molecular chaperone. Proc. Natl Acad. Sci. USA 89, 10449–10453 (1992).
Sun, Y. & MacRae, T. H. The small heat shock proteins and their role in human disease. FEBS J. 272, 2613–2627 (2005).
Carra, S., Sivilotti, M., Zobel, A. T. C., Lambert, H. & Landry, J. HspB8, a small heat shock protein mutated in human neuromuscular disorders, has in vivo chaperone activity in cultured cells. Hum. Mol. Genet. 14, 1659–1669 (2005).
Carra, S. et al. Different anti-aggregation and pro-degradative functions of the members of the mammalian sHSP family in neurological disorders. Philos. Trans. R. Soc. B Biol. Sci. 368, 1–13 (2013).
Vendredy, L., Adriaenssens, E. & Timmerman, V. Small heat shock proteins in neurodegenerative diseases. Cell Stress Chaperones 25, 679–699 (2020).
Michaud, S., Marin, R. & Tanguay, R. M. Regulation of heat shock gene induction and expression during Drosophila development. Cell. Mol. Life Sci. 53, 104–113 (1997).
Morrow, G. & Tanguay, R. M. Small heat shock protein expression and functions during development. Int. J. Biochem. Cell Biol. 44, 1613–1621 (2012).
Reinle, K., Mogk, A. & Bukau, B. The diverse functions of small heat shock proteins in the proteostasis network: functions and mechanisms of sHsps. J. Mol. Biol. 434, 167157 (2022).
Sun, W., Bernard, C., Van De Cotte, B., Van Montagu, M. & Verbruggen, N. At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J. 27, 407–415 (2001).
Jiang, C. et al. A cytosolic class i small heat shock protein, RcHSP17.8, of Rosa chinensis confers resistance to a variety of stresses to Escherichia coli, yeast and Arabidopsis thaliana. Plant, Cell Environ. 32, 1046–1059 (2009).
Sato, Y. & Yokoya, S. Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17.7. Plant Cell Rep. 27, 329–334 (2008).
Feng, X. H. et al. A small heat shock protein CaHsp25.9 positively regulates heat, salt, and drought stress tolerance in pepper (Capsicum annuum L.). Plant Physiol. Biochem. 142, 151–162 (2019).
Clegg, J. S., Jackson, S. A. & Warner, A. H. Extensive intracellular translocations of a major protein accompany anoxia in embryos of Artemia franciscana. Exp. Cell Res. 212, 77–83 (1994).
Liang, P., Amons, R., Clegg, J. S. & MacRae, T. H. Molecular characterization of a small heat shock/α-crystallin protein in encysted Artemia embryos. J. Biol. Chem. 272, 19051–19058 (1997).
Gusev, O., Cornette, R., Kikawada, T. & Okuda, T. Expression of heat shock protein-coding genes associated with anhydrobiosis in an African chironomid Polypedilum vanderplanki. Cell Stress Chaperones 16, 81–90 (2011).
Wang, C., Grohme, M. A., Mali, B., Schil, R. O. & Frohme, M. Towards decrypting cryptobiosis—analyzing anhydrobiosis in the tardigrade Milnesium tardigradum using transcriptome sequencing. PLoS One 9, 1–15 (2014).
Erkut, C. et al. Molecular strategies of the Caenorhabditis elegans dauer larva to survive extreme desiccation. PLoS One 8, 1–19 (2013).
Jackson, S. A. & Clegg, J. S. Ontogeny of low molecular weight stress protein p26 during early development of the brine shrimp, Artemia franciscana. Dev. Growth Differ. 38, 153–160 (1996).
Liang, P. & MacRae, T. H. The synthesis of a small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev. Biol. 207, 445–456 (1999).
Liang, P., Amons, R., Macrae, T. H. & Clegg, J. S. Purification, structure and in vitro molecular-chaperone activity of Artemia p26, a small heat-shock/α-crystallin protein. Eur. J. Biochem. 243, 225–232 (1997).
Ma, X. et al. A small stress protein acts synergistically with trehalose to confer desiccation tolerance on mammalian cells. Cryobiology 51, 15–28 (2005).
Schokraie, E. et al. Investigating heat shock proteins of tardigrades in active versus anhydrobiotic state using shotgun proteomics. J. Zool. Syst. Evol. Res. 49, 111–119 (2011).
Beltrán-Pardo, E., Jönsson, K. I., Harms-Ringdahl, M., Haghdoost, S. & Wojcik, A. Tolerance to gamma radiation in the tardigrade hypsibius dujardini from embryo to adult correlate inversely with cellular proliferation. PLoS One 10, 1–13 (2015).
Seki, K. & Toyoshima, M. Preserving tardigrades under pressure. Nature 395, 853–854 (1998).
Jönsson, K. I., Rabbow, E., Schill, R. O., Harms-Ringdahl, M. & Rettberg, P. Tardigrades survive exposure to space in low Earth orbit. Curr. Biol. 18, 729–731 (2008).
Förster, F. et al. Tardigrade workbench: comparing stress-related proteins, sequence-similar and functional protein clusters as well as RNA elements in tardigrades. BMC Genomics 10, 469 (2009).
Hashimoto, T. et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 7, 12808 (2016).
Boothby, T. et al. Tardigrades use intrinsically disordered proteins to survive desiccation. Mol. Cell 65, 975–984 (2017).
Yoshida, Y. et al. Comparative genomics of the tardigrades Hypsibius dujardini and Ramazzottius varieornatus. PLoS Biol. 15, e2002266 (2017).
Kamilari, M., Jørgensen, A., Schiøtt, M. & Møbjerg, N. Comparative transcriptomics suggest unique molecular adaptations within tardigrade lineages. BMC Genomics 20, 1–19 (2019).
de la Fuente, M. & Novo, M. Understanding diversity, evolution, and structure of small heat shock proteins in annelida through in silico analyses. Front. Physiol. 13, 1–15 (2022).
Wu, J., Wang, M., Zhou, L. & Yu, D. Small heat shock proteins, phylogeny in filamentous fungi and expression analyses in Aspergillus nidulans. Gene 575, 675–679 (2016).
Zhang, T., Li, G. R., Yang, Z. J. & Nevo, E. Adaptive evolution of duplicated hsp17 genes in wild barley from microclimatically divergent sites of Israel. Genet. Mol. Res. 13, 1220–1232 (2014).
Nicosia, A. et al. Characterization of small hsps from Anemonia viridis reveals insights into molecular evolution of alpha crystallin genes among cnidarians. PLoS One 9, 1–15 (2014).
Zhang, K. et al. A novel mechanism for small heat shock proteins to function as molecular chaperones. Sci. Rep. 5, 1–8 (2015).
Sun, Y., Mansour, M., Crack, J. A., Gass, G. L. & MacRae, T. H. Oligomerization, chaperone activity, and nuclear localization of p26, a small heat shock protein from Artemia franciscana. J. Biol. Chem. 279, 39999–40006 (2004).
Kim, K. K., Kim, R. & Kim, S. H. Crystal structure of a small heat-shock protein. Nature 394, 595–599 (1998).
Stengel, F. et al. Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc. Natl Acad. Sci. USA 107, 2007–2012 (2010).
Waters, E. R. & Vierling, E. Plant small heat shock proteins—evolutionary and functional diversity. N. Phytol. 227, 24–37 (2020).
Åhrman, E., Gustavsson, N., Hultschig, C., Boelens, W. C. & Emanuelsson, C. S. Small heat shock proteins prevent aggregation of citrate synthase and bind to the N-terminal region which is absent in thermostable forms of citrate synthase. Extremophiles 11, 659–666 (2007).
Mymrikov, E. V., Daake, M., Richter, B., Haslbeck, M. & Buchner, J. The chaperone activity and substrate spectrum of human small heat shock proteins. J. Biol. Chem. 292, 672–684 (2017).
Piszkiewicz, S. et al. Protecting activity of desiccated enzymes. Protein Sci. 28, 941–951 (2019).
Boswell, L. C., Menze, M. A. & Hand, S. C. Group 3 late embryogenesis abundant proteins from embryos of Artemia franciscana: structural properties and protective abilities during desiccation. Physiol. Biochem. Zool. 87, 640–651 (2014).
Shrivastava, A. et al. The cytoprotective sequestration activity of small heat shock proteins is evolutionarily conserved. J. Cell Biol. 221, e202202149 (2022).
Carra, S. et al. Small heat shock proteins: multifaceted proteins with important implications for life. Cell Stress Chaperones 24, 295–308 (2019).
Reuner, A. et al. Stress response in tardigrades: differential gene expression of molecular chaperones. Cell Stress Chaperones 15, 423–430 (2010).
Bar-Lavan, Y. et al. A differentiation transcription factor establishes muscle-specific proteostasis in Caenorhabditis elegans. PLoS Genet. 12, 1–27 (2016).
Tiago, T. et al. Small heat-shock protein HSPB3 promotes myogenesis by regulating the lamin B receptor. Cell Death Dis. 12, 1–19 (2021).
Neves, R. C., Hvidepil, L. K. B., Sørensen-Hygum, T. L., Stuart, R. M. & Møbjerg, N. Thermotolerance experiments on active and desiccated states of Ramazzottius varieornatus emphasize that tardigrades are sensitive to high temperatures. Sci. Rep. 10, 1–12 (2020).
Hengherr, S., Worland, M. R., Reuner, A., Brümmer, F. & Schill, R. O. High‐temperature tolerance in anhydrobiotic tardigrades is limited by glass transition. Physiol. Biochem. Zool. 82, 749–755 (2009).
Malkeyeva, D., Kiseleva, E. & Fedorova, S. Small heat shock protein Hsp67Bc plays a significant role in Drosophila melanogaster cold stress tolerance. J. Exp. Biol. 223, jeb219592 (2020).
Haslbeck, M., Franzmann, T., Weinfurtner, D. & Buchner, J. Some like it hot: the structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 12, 842–846 (2005).
Peschek, J. et al. The eye lens chaperone α-crystallin forms defined globular assemblies. Proc. Natl Acad. Sci. USA 106, 13272–13277 (2009).
Baranova, E. V. et al. Three-dimensional structure of α-crystallin domain dimers of human small heat shock proteins HSPB1 and HSPB6. J. Mol. Biol. 411, 110–122 (2011).
Delbecq, S. P. & Klevit, R. E. One size does not fit all: the oligomeric states of αb crystallin. FEBS Lett. 587, 1073–1080 (2013).
den Engelsman, J. et al. The small heat-shock proteins HSPB2 and HSPB3 form well-defined heterooligomers in a unique 3 to 1 subunit ratio. J. Mol. Biol. 393, 1022–1032 (2009).
Mymrikov, E. V. et al. Regulation of small heat-shock proteins by hetero-oligomer formation. J. Biol. Chem. 295, 158–169 (2020).
Day, R. M., Gupta, J. S. & MacRae, T. H. A small heat shock/α-crystallin protein from encysted Artemia embryos suppresses tubulin denaturation. Cell Stress Chaperones 8, 183–193 (2003).
Tsvetkova, N. M. et al. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl Acad. Sci. USA 99, 13504–13509 (2002).
Gusev, N. B., Bogatcheva, N. V. & Marston, S. B. Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochem 67, 511–519 (2002).
Capozzi, V. et al. Inactivation of a small heat shock protein affects cell morphology and membrane fluidity in Lactobacillus plantarum WCFS1. Res. Microbiol. 162, 419–425 (2011).
Carra, S., Seguin, S. J., Lambert, H. & Landry, J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J. Biol. Chem. 283, 1437–1444 (2008).
Kamradt, M. C., Chen, F., Sam, S. & Cryns, V. L. The small heat shock protein αB-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J. Biol. Chem. 277, 38731–38736 (2002).
Morelli, F. F. et al. Aberrant compartment formation by HSPB2 mislocalizes lamin A and compromises nuclear integrity and function. Cell Rep. 20, 2100–2115 (2017).
Lu, S. et al. Heat-shock chaperone HSPB1 regulates cytoplasmic TDP-43 phase separation and liquid-to-gel transition. Nat. Cell Biol. 24, 1378–1393 (2022).
Liu, Z. et al. Hsp27 chaperones FUS phase separation under the modulation of stress-induced phosphorylation. Nat. Struct. Mol. Biol. 27, 363–372 (2020).
Boczek, E. E. et al. HspB8 prevents aberrant phase transitions of FUS by chaperoning its folded RNA binding domain. Elife 10, 1–27 (2021).
Belott, C., Janis, B. & Menze, M. A. Liquid–liquid phase separation promotes animal desiccation tolerance. Proc. Natl. Acad. Sci. USA 117 202014463 (2020).
Ishida, T. & Kinoshita, K. PrDOS: Prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res. 35, 460–464 (2007).
Lu, S. et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 48, D265–D268 (2020).
Boothby, T. C. Total RNA extraction from tardigrades. Cold Spring Harb. Protoc. 2018, 905–907 (2018).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009).
Cole, D., Young, G., Weigel, A., Sebesta, A. & Kukura, P. Label-free single-molecule imaging with numerical-aperture-shaped interferometric scattering microscopy. ACS Photonics 4, 211–216 (2017).
Young, G. et al. Quantitative mass imaging of single biological macromolecules. Science 360, 423–427 (2018).
Acknowledgements
We thank Nathan Nicely of the Protein Expression and Purification core facility at UNC for purification of HSP17, HSP21, and HSP24.6 and for training on the mass photometer. We thank Kristen White and Kathleen A. Clardy of the Microscopy Services Laboratory for sample preparation and TEM imaging. We are grateful to Roy A. Quinlan and Muhammad Alansari for helpful discussions. This work was supported by the National Institutes of Health (F32GM131577, awarded to J.D.H.), the National Science Foundation (IOS 2028860, awarded to BG), and MIUR (Departments of excellence 2018-2022, E91I18001480001, awarded to S.C). The Protein Expression and Purification core facility at UNC is supported by the National Cancer Institute of the National Institutes of Health under award number P30CA016086. The Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, is supported in part by P30CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.D.H.: Conceptualization, formal analysis, investigation, writing – original draft, writing – review and editing, visualization, project administration, funding acquisition. S.C.: Conceptualization, writing – review and editing, supervision, funding acquisition. B.G.: Conceptualization, writing – review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Eve Rogers. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hibshman, J.D., Carra, S. & Goldstein, B. Tardigrade small heat shock proteins can limit desiccation-induced protein aggregation. Commun Biol 6, 121 (2023). https://doi.org/10.1038/s42003-023-04512-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04512-y
This article is cited by
-
Elevated external temperature affects cell ultrastructure and heat shock proteins (HSPs) in Paramacrobiotus experimentalis Kaczmarek, Mioduchowska, Poprawa, & Roszkowska, 2020
Scientific Reports (2024)
-
A review on oligomeric polydispersity and oligomers-dependent holding chaperone activity of the small heat-shock protein IbpB of Escherichia coli
Cell Stress and Chaperones (2023)
-
The beauty and complexity of the small heat shock proteins: a report on the proceedings of the fourth workshop on small heat shock proteins
Cell Stress and Chaperones (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.