Abstract
Hematopoietic stem cells (HSCs) are produced from the blood vessel walls and circulate in the blood during the perinatal period. However, the migration dynamics of how HSCs enter the bone marrow remain elusive. To observe the dynamics of HSCs over time, the present study develops an intravital imaging method to visualize bone marrow in neonatal long bones formed by endochondral ossification which is essential for HSC niche formation. Endogenous HSCs are labeled with tdTomato under the control of an HSC marker gene Hlf, and a customized imaging system with a bone penetrating laser is developed for intravital imaging of tdTomato-labeled neonatal HSCs in undrilled tibia, which is essential to avoid bleeding from fragile neonatal tibia by bone drilling. The migration speed of neonatal HSCs is higher than that of adult HSCs. Neonatal HSCs migrate from outside to inside the tibia via the blood vessels that penetrate the bone, which is a transient structure during the neonatal period, and settle on the blood vessel wall in the bone marrow. The results obtained from direct observations in vivo reveal the motile dynamics and colonization process of neonatal HSCs during bone marrow formation.
Similar content being viewed by others
Introduction
Hematopoietic stem cells (HSCs) are characterized by multilineage differentiation and the capacity for self-renewal to sustain lifelong hematopoiesis1,2. HSCs actively divide with a high metabolic status in the neonatal stage3,4 and then show a quiescent state with decreased metabolic status to maintain HSCs by preventing excess cell division in the adult stage5. Transcriptional HSC profiles are dramatically changed during the perinatal period6 and these alternations to HSC status could be related to their microenvironment, known as their niche7, during development. Previous studies have suggested that HSC precursors are generated from the aorta–gonad–mesonephros region during the embryonic period and then transfer to the fetal liver, where they mature into definitive HSCs. HSCs are thought to migrate to the bone marrow niche during the perinatal period8,9. However, the migration dynamics of HSCs during early development remain unclear in living animals.
The dynamics of HSCs have been studied in adult mice using intravital imaging. Fluorescent-labeled HSCs have been transplanted into irradiated mice to observe HSC dynamics in vivo, and intravital imaging of the bone marrow has been performed in the calvarium10,11,12,13 or drilled long bones14,15,16,17. However, irradiation prior to transplantation could affect the dynamics of HSCs by changing the surrounding microenvironment18,19,20. Therefore, transplantation of large numbers of HSCs into mice that have not been irradiated was conducted to avoid the irradiation effect21. Furthermore, reporter mice have been developed to selectively label HSCs. Long bone imaging after optical clearing in α-catulinGFP mice suggests that HSCs mainly reside in the perisinusoidal niche22. The Mds1GFP/+Flt3Cre system allows accurate identification of long-term HSCs through deletion of green fluorescent protein (GFP) in progenitor cells, which enables intravital imaging of endogenous long-term HSCs in the calvarium bone marrow in adult mice10.
Despite over a decade of years of research into intravital imaging of bone marrow, the dynamics of HSCs in the neonatal stage remain unexplored. The present study established a method for imaging endogenous HSCs within the bone marrow of tibial bones in adult and neonatal mice without damaging the bone. The tibial bone is formed by endochondral ossification, which is important for the formation of the HSC niche23, although the calvarium is formed via intramembranous ossification24. Therefore, we focused on imaging the bone marrow of the tibia rather than that of the calvarium. Hlf-tdTomato knock-in (KI) mice25, in which a red fluorescent protein tdTomato is expressed under the promoter control of the HSC marker gene Hlf26,27, was used for intravital imaging of endogenous HSCs in the tibia. The findings of the present study revealed the changes in the dynamics of HSCs within the bone marrow between the adult and neonatal stages and the process of HSC homing to the bone marrow during the neonatal period.
Results
Cells with the highest Hlf-tdTomato expression levels have bone marrow reconstitution capacity
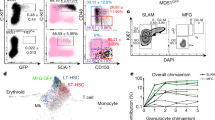
We previously reported that HSCs have higher levels of tdTomato than other hematopoietic progenitors in the fetal livers of Hlf-tdTomato KI mice25. Transplantation experiments were performed to confirm the stem cell potential of bone marrow cells with higher levels of Hlf-tdTomato in the bone marrow of the tibia. Flow cytometry analysis showed that 47.0 ± 4.4% of whole bone marrow cells from adult long bones of Hlf-tdTomato KI mice were positive for CD45, which is a panhematopoietic marker (Fig. 1a, left panel, n = 4). Cells with the top 0.011% tdTomato intensity within the CD45-positive cells (0.0049% of the whole bone marrow cells) were defined as Hlf-tdTomatohi cells (Fig. 1b). All the Hlf-tdTomatohi cells were located within the Sca-1+c-Kit+ fraction, including phenotypic HSCs (CD150+CD48−) and short-term HSCs (CD150−CD48−) (Fig. 1a, right panel and Supplementary Fig. 1). To determine whether Hlf-tdTomatohi cells show a high frequency of functional HSCs within the bone marrow of the tibia, we compared engraftment capacity between the Hlf-tdTomatohi and Hlf-tdTomato− cells (44.3% of the whole bone marrow cells) by the transplantation assay (Fig. 1b). A total of 100 Hlf-tdTomatohi cells were capable of engraftment after primary and secondary transplantation, whereas 5000 Hlf-tdTomato− cells were not capable (Fig. 1c, d). These results indicate that functional HSCs were enriched within the Hlf-tdTomatohi fraction in the bone marrow of the long bones.
a Hlf-tdTomato expression in the bone marrow blood cells obtained from long bones. The black box indicates the Hlf-tdTomatohi population. b Flow cytometry sorting of Hlf-tdTomato cells. The Hlf-tdTomatohi box population was used for transplantation experiments. The tdTomato− box population was used as a negative control. c Bone marrow transplantation (BMT) experiments. Irradiated mice were transplanted with 100 tdTomatohi/CD45+ cells or 5000 tdTomato−/CD45+ cells. d Second BMT experiments.
Intravital imaging enables observation of endogenous HSCs in the bone marrow of undrilled long bones
Next, we developed a method to observe the dynamics of Hlf-tdTomatohi HSCs in the tibial bone marrow in vivo (Fig. 2). Previous studies have used drilled tibia for intravital imaging of HSCs in the long bones14,15,16,17. However, drilling of the long bones precluded the comparison of the HSC dynamics between adults and neonates for two reasons. First, drilling could disturb the microenvironment of the bone marrow, and second, the long bone of neonates is too fragile to be drilled, and it is not possible to avoid bleeding from the blood vessels penetrating the neonatal tibia (Supplementary Fig. 2a and Supplementary Movie 1). A multiphoton imaging system equipped with a bone-penetrating fiber laser (average power, >2 W; pulse width, 55> fs; wavelength, 1070 nm) was established to overcome the limitations of the conventional methods (Fig. 2a). In our system, tdTomato-positive cells were observed under the intact tibial bone tissue, which was visualized with second harmonic generation (SHG) signals in adult mice (3 months old) in vivo (Fig. 2b; Supplementary Movie 2). Blood capillaries in the bone marrow were visualized by intravenous injection of an infrared fluorescent dye Qtracker 655 to confirm the location of the tdTomato-positive cells within the tibial bone marrow. Intravital imaging showed that tdTomato-positive cells were located around the blood capillary network (Fig. 2c, d), which are typical blood vessel patterns in the bone marrow of long bones28. These results suggest that Hlf-tdTomato-positive cells in the intact tibial bone marrow can be observed by the method developed in the present study.
a Experimental schema of intravital imaging of the tibial bone. The tibial bone was exposed, and the bone marrow was imaged without bone drilling using a high-powered infrared laser. b Z-stack images obtained via in vivo imaging of the bone marrow in the undrilled tibia of Hlf-tdTomato KI mice. Hlf-tdTomato-positive cells were observed in the bone marrow cavity. Bone surface, bone, and bone marrow cavities. The depth is indicated in the upper right corner of each panel. SHG, second harmonic generation. c Orthogonal view of 3D images confirmed that Hlf-tdTomato-positive cells were in the bone marrow under the tibial bone. Blood capillaries in the tibial bone marrow were visualized by intravenous injection of Qtracker 655. Cells indicated with arrows 1 and 2 are shown in higher magnification in (d). See also Supplementary Movie 2. d Higher magnification images of Hlf-tdTomato-positive cells located around the bone marrow capillary. e Intravital imaging of a Runx1-GFP mouse before drilling. Depth is 155 μm from the bone surface. f Intravital imaging of the Runx1-GFP mouse after drilling. The same region in (d) was reimaged after drilling. Background signals were imaged in the red channel, suggesting that most signals in the green channel were artificial backgrounds. Arrows indicate Runx1-GFP cells that had no background signals in the red channel.
The technical advances of our method were evaluated by comparing it with the conventional method. For the conventional method, intravital imaging of Runx1-GFP transgenic mice, in which HSCs and progenitor cells strongly express GFP29, was conducted using a short wavelength (920 nm) laser which does not easily penetrate bone. Intravital imaging showed that Runx1-GFP labeled cells in the bone marrow were blurred without drilling (Fig. 2e), and GFP signals were only clearly observed after drilling (Fig. 2f), indicating that drilling is essential for the conventional method. As previously reported11, artificial background signals were observed in the green channel (Fig. 2f), whereas these were rarely seen using our imaging method (Fig. 2b). These results highlight the advances of the intravital imaging developed in the present study.
There were 18.2 ± 1.0 tdTomato-positive cells in the volume of the intravital images (around 600 μm × 600 μm × 100 μm = 3.6 × 107 μm3; n = 5 volumes from five mice). Immunohistochemical staining of the tibial bone sections obtained from Hlf-tdTomato KI mice was conducted (Fig. 3a) to determine which tdTomato-positive cells in the intravital images corresponded to the Hlf-tdTomatohi HSCs that were identified in the flow cytometry analysis (Fig. 1). In the histological sections of the tibial bone, there were 18,201 ± 933 CD45-positive cells and 1,630 ± 269 tdTomato-positive cells within the same volume as the in vivo-imaged volume (n = 3 sections from three mice; Fig. 3b, c). Since the cells with the top 0.011% tdTomato intensity in CD45-positive cells were defined as Hlf-tdTomatohi cells (Fig. 1), those with the top two tdTomato fluorescent intensities in the in vivo images corresponded to Hlf-tdTomatohi cells (18,201 cells × 0.011% = 2 cells; Fig. 3c). We defined the remaining Hlf-tdTomato-positive cells with lower intensity in the intravital images that include differentiated progenitor cells25 as Hlf-tdTomatolow cells (16 cells in Fig. 3c), which had lower stem cell potential (Supplementary Fig. 3). Most of the tdTomato-positive cells in the histological sections were not visible using intravital imaging due to the very low intensity (1612 cells in Fig. 3c). These results suggested that the cells with top two tdTomato fluorescent intensities in the in vivo image were HSCs.
a Immunohistochemical staining of tibial bone sections was obtained from an adult Hlf-tdTomato KI mouse. Ter-119, red blood cell marker; CD45, blood cell marker except for mature red blood cells and platelets; DAPI, nuclear marker. b Fluorescence distribution of tdTomato signals in CD45-positive cells in the histological sections of the tibial bone. The number of CD45-positive cells within the same volume as the in vivo-imaged volume was 18,201 ± 933 cells. Error bars indicate standard error. c The number of CD45 and tdTomato-double-positive cells within the same volume as the in vivo-imaged volume was 1630 ± 269 cells. The number of tdTomato-positive cells was 18.2 ± 1.0 cells, suggesting that the remaining 1612 cells were not visible in the intravital images due to the very low fluorescent intensity. The cells with the top 0.011% tdTomato intensity in CD45-positive cells were defined as Hlf-tdTomatohi cells (Fig. 1); therefore, the cells with the top two tdTomato fluorescent intensities in the in vivo images correspond to the Hlf-tdTomatohi cells (18,201 cells × 0.011% = 2 cells). We defined the remaining Hlf-tdTomato-positive cells with lower fluorescent intensity (16 cells in average) as Hlf-tdTomatolow cells for quantitative analysis of Hlf-tdTomato-positive cell dynamics in Figs. 4 and 5.
Intravital imaging of intact tibia reveals endogenous HSCs are stationary in adult bone marrow
Three-dimensional time-lapse imaging of undrilled tibial bone marrow was performed to observe the in vivo dynamics of Hlf-tdTomatohi HSCs (Fig. 4a and Supplementary Movie 3). Artifactual movement of the image area, mainly caused by the heartbeat, was corrected using image registration. Hlf-tdTomatohi HSCs were stationary (Fig. 4b), although they showed oscillatory movements in the restricted area. In contrast, Hlf-tdTomatolow cells migrated (Fig. 4c). Quantitative analysis of the HSC migration using TrackMate30 revealed that the velocity of the Hlf-tdTomatohi HSCs (0.096 ± 0.019 µm/min, 10 cells from five mice) was significantly lower than that of Hlf-tdTomatolow cells (0.169 ± 0.017 µm/min, 81 cells from five mice; p = 0.008; t = 2.886; g = 0.505; Fig. 4d). We also showed long-term engraftment of Hlf-tdTomatohi cells (Fig. 1c, d and Supplementary Fig. 1), indicating that HSCs were stationary but oscillatory. However, differentiated cells were motile in the bone marrow of adult long bones. Therefore, our findings demonstrate that Hlf-tdTomato KI mice, the “inside-bone intravital imaging system” and quantitative bioimaging analysis facilitate the evaluation of the migration dynamics of endogenous HSCs in the native microenvironment of long bones.
a Hlf-tdTomato-positive cells in the tibial bone marrow were imaged for 2 h in vivo. Arrows indicate Hlf-tdTomatohi HSCs and arrowheads indicate Hlf-tdTomatolow cells. See also Supplementary Movie 3. b Higher magnification time-lapse images of Hlf-tdTomatohi HSCs. c Higher magnification time-lapse images of Hlf-tdTomatolow cells. d Quantitative comparison of the migration dynamics between Hlf-tdTomatohi cells (ten cells from five mice) and Hlf-tdTomatolow cells (81 cells from five mice).
Changes in expression of cell migration-related genes in neonatal HSCs
Neonatal HSCs are characterized by fast cell cycling and higher mitochondrial membrane potential4, indicating changes in the cellular properties between adult and neonatal HSCs. Gene expression patterns were compared between developing HSCs and matured HSCs using RNA-seq to evaluate changes in the properties of neonatal HSCs.
Gene set enrichment analysis (GSEA) showed significant enrichment in cell migration-related genes in neonatal HSCs (Supplementary Fig. 4a, b). Consistently, changes in the expression of genes related to the cytoskeleton and cell adhesion were observed in neonatal HSCs (Supplementary Fig. 4c–f). Differences in the cell migration-related genes indicated differences in the migration dynamics of neonatal and adult HSCs in the tibial bone marrow. From these results, we focused on the migration dynamics in subsequent experiments for intravital imaging of neonatal mice.
Migration of Hlf-tdTomatohi cells in the neonatal tibial bone marrow and bone cavity
Migration of HSCs into the bone marrow from other hematopoietic organs has been hypothesized since adult-type definitive HSCs are generated from the aorta–gonad–mesonephros region8,25. However, the migration dynamics of HSCs during development is unclear. Therefore, three-dimensional time-lapse imaging of the bone marrow was performed in the undrilled tibia in neonates (postnatal day 2; Fig. 5a, left). The orthogonal view confirmed that the intravital imaging of the intact tibial bone marrow enabled the observation of endogenous tdTomato-positive cells in neonatal Hlf-tdTomato KI mice (Fig. 5a, right; Supplementary Movie 4). Quantitative analyses showed that the velocity of Hlf-tdTomatohi cells (1.516 ± 1.010 µm/min, six cells from three mice; top two fluorescent intensities) was much higher than that of Hlf-tdTomatolow cells (0.078 ± 0.010 µm/min, 24 cells from three mice; p = 0.002; Z = 3.059; r = 0.558) in neonates (Fig. 5b). Furthermore, the velocity of Hlf-tdTomatohi cells in neonates was much higher than that in adults (p = 0.017; Z = 2.386; r = 0.597, Supplementary Fig. 2b). These results suggest that HSCs are motile in the tibial bone marrow of neonates.
a Tibial bone was exposed on postnatal day 2 (left). Orthogonal view image of the tibial bone marrow (right). See also Supplementary Movie 4. b Quantitative comparison of the migration dynamics between Hlf-tdTomatohi cells (six cells from three mice) and Hlf-tdTomatolow cells (24 cells from three mice) in neonates. c Time-lapse images of developing (P2) bone marrow showing migration of an Hlf-tdTomatohi cell in the blood vessels. White arrows indicate migrating cells in the blood vessel and yellow arrows indicate stable cells outside the blood vessels. See also Supplementary Movie 5. d Another example of an Hlf-tdTomatohi cell migrating in the bone marrow blood vessels in a neonatal mouse (P1).
Intravital imaging was conducted after visualizing blood vessels by injecting Qtracker 655 via the superficial temporal vein31 to check whether the motile Hlf-tdTomatohi cells were extravascular or intravascular. Some of the Hlf-tdTomatohi cells rapidly migrated in the blood vessels (Fig. 5c, d, white arrows). Moreover, Hlf-tdTomatohi cell attached to the vessels during the imaging session, indicating extravasation and homing to the bone marrow (Fig. 5c, 63 and 100 min; Supplementary Movie 5). In contrast, Hlf-tdTomatohi cells located outside the capillaries, which appeared to be extravasated prior to the imaging session, were stationary (Fig. 5c, yellow arrows). These results suggest that motile Hlf-tdTomatohi cells migrate in the blood vessels of the neonatal tibia.
Finally, migration of HSCs from outside the bone marrow was observed using time-lapse imaging of the tibial bone cavity, where the blood vessels that penetrate the bone are located (Fig. 6a). An Hlf-tdTomatohi cell was observed to rapidly migrate within the bone cavities (Fig. 6b and Supplementary Movie 6). Interestingly, the distance between the observed cell and the inner bone surface appeared to be increased (Supplementary Fig. 5a, b), suggesting migration of the cell to the deeper part of the bone. Taken together, these results indicate that HSCs migrate in the bone cavities and bone marrow during tibial bone marrow formation.
a Two-photon images showing the blood vessels penetrating the bone cavity in the tibial bone in a neonatal mouse (P3). Yellow arrows indicate bone cavities. b Time-lapse images of Hlf-tdTomatohi cells migrating from the bone surface to the bone marrow cavity. White arrows indicate a migrating cell in the bone cavity. See also Supplementary Movie 6. c “Transient vessel penetration model” for HSC homing during bone marrow formation. HSCs migrate from outside the tibia to inside via transient blood vessels penetrating the bone during bone marrow formation.
Discussion
An intravital imaging method for endogenous HSCs in the bone marrow of long bones was established without drilling using a customized multiphoton imaging system. This system was used to reveal that HSCs within the bone marrow of long bones in adult mice are stationary but oscillatory. In contrast, HSCs in neonatal mice were found to be motile within the bone marrow of the long bones and showed differential dynamic behavior from adult HSCs. GSEA consistently demonstrated enrichment of cell migration-related genes in neonatal HSCs. Neonatal HSCs migrate to the bone marrow via vessels that penetrate the cavities in immature tibial bone (Fig. 6c), which rarely exist in the adult tibia (Fig. 2b). Thus, the results of the present study provide evidence for HSC migration to the bone marrow at the early stage of lifelong hematopoiesis before HSCs become quiescent in the cell cycle, which has been hypothesized8 but has not been directly verified in living animals.
The intravital imaging described in the present study enables the observation of endogenous HSCs in native microenvironments of undrilled long bones (Figs. 2, 4, 5, 6 and Supplementary Movies 2–6). This imaging approach has two important advantages. First, intravital imaging of bone marrow in the long bones does not require drilling. To date, previous studies have reported intravital imaging of bone marrow in the calvarium and drilled long bones10,11,12,13,16. Although intravital imaging of bone marrow in the calvarium can be achieved without drilling, the calvarium is formed by intramembranous ossification. Endochondral ossification is required for the formation of the HSC niche and progenitors from the calvarium do not efficiently form an HSC niche;23 therefore, intravital imaging in long bones is essential to evaluate neonatal HSC migration dynamics in native microenvironments. Furthermore, it was necessary to establish intravital imaging of long bones without drilling in neonates because bleeding is unavoidable when neonatal long bones are drilled (Supplementary Fig. 2a and Supplementary Movie 1). In the present study, a bone-penetrating laser (high power at a long-wavelength) for a red fluorescent protein tdTomato enabled imaging of HSCs in the bone marrow without bone drilling in vivo (Fig. 2 and Supplementary Movie 2). The advantage of our approach is shown by the clearer images and much lower background signals of Hlf-tdTomatohi cells (Fig. 2b–d), compared with those of GFP-positive cells observed by intravital imaging of Runx1-GFP transgenic mice (Fig. 2e, f). The second advantage is the observation of endogenous HSCs, which is enabled by Hlf-tdTomato KI mice. Previous studies have mainly analyzed the dynamics of transplanted HSCs. However, it is unclear whether the dynamics of exogenous HSCs injected into irradiated mice is the same as those of endogenous HSCs in a steady-state18,19,20. In the present study, HSC dynamics were observed in native microenvironments of neonatal bone marrow without radiation. Compared with imaging of endogenous HSCs using α-catulinGFP mice after bone clearing22 and intravital imaging of endogenous HSCs in the bone marrow of calvarium by Mds1GFP/+Flt3Cre system10, our imaging approach is unique in intravital imaging of endogenous HSCs in undrilled long bones, which is essential for studying the dynamics of HSCs in neonates.
Intravital imaging has revealed a biological characteristic, namely motile dynamics, of neonatal HSCs (Fig. 5, Supplementary Fig. 2b, and Supplementary Movies 4–6) in addition to neonatal HSC properties, such as active cell cycling and high metabolic status. The migration speed of neonatal Hlf-tdTomatohi cells was higher than that of adult Hlf-tdTomatohi cells, and neonatal Hlf-tdTomatohi cells included cells migrating in blood vessels. Interestingly, the velocity of an Hlf-tdTomatohi cell (Fig. 5b) was much slower than the velocity of blood flow in the calvarial sinusoids32. Therefore, neonatal HSCs may not passively flow in the vessels but may actively home to the bone marrow vessels. Taken together with the RNA-seq results in the present study regarding differences in expression of gene related to cell migration, cytoskeleton, and cell adhesion between developing and mature HSCs (Supplementary Fig. 4), these results suggest that neonatal HSCs have different dynamic properties from adult HSCs to migrate between distinct hematopoietic organs during development. On the other hand, adult HSCs in the bone marrow of long bones were stationary but shown to oscillate in the present study (Fig. 4 and Supplementary Movie 3). In agreement with this finding, HSCs from adult mice without infection have lower linear progression (displacement/total track length) than those with acute infection, whereas adult HSCs oscillate in the bone marrow of irradiated recipient mice13. HSC oscillation may reflect dynamic interaction between HSCs and niche cells.
Intravital imaging showed that the tibias of neonates contained holes through which blood vessels passed (Fig. 6a), and neonatal HSCs migrated through the blood vessels and settled on the blood vessels in the bone marrow (Figs. 5c, d, 6b, Supplementary Fig. 5, and Supplementary Movies 5, 6). In contrast, intravital imaging demonstrated that such holes and blood vessels penetrating the bones rarely exist in the matured tibia of adults (Fig. 2b). From these results, we propose a “transient vessel penetration model” for HSC homing during bone marrow formation (Fig. 6c). In mice, long bones have perfused vasculatures at around embryonic day 16.5 after endochondral ossification31. Transient blood vessels penetrating the immature long bones and bone marrow niche formed after endochondral ossification may confer an optimal environment for HSC migration into the bone marrow from other hematopoietic organs. Similarly, in mouse liver, the transition of portal vessels from Neuropilin-1+Ephrin-B2+ artery to EphB4+ vein phenotype at birth is associated with HSC release from the portal vessels7.
In conclusion, we developed an intravital imaging method to examine endogenous HSCs in the native microenvironments of the bone marrow without drilling long bones formed by endochondral ossification, which is essential for HSC niche formation. The next challenge is to increase the success rate of neonatal imaging. This can be overcome by improving the intravenous injection method required for blood vessel visualization, and refining the imaging conditions such as anesthesia. The strategy would further elucidate the dynamics of endogenous HSC migration between native niches in distinct hematopoietic organs during development and the mechanisms of HSC migration abnormalities in diseases.
Methods
Animals
Hlf-tdTomato KI mice were generated in the previously study25. Runx1-GFP mice were kindly provided by M. Osato (Cancer Science Institute of Singapore, National University of Singapore)29. C57BL/6-Ly5.1 mice were used for competitive bone marrow transplantation experiments. Data were taken from distinct mice or cells unless otherwise noted. All animal experiments were conducted according to the guidelines of Kumamoto University on animal use.
Flow cytometry
For flow cytometric analysis, bone marrow cells were prepared by crushing the bones using a mortar and pestle25. Antibodies against CD45 (30-F11, 1:100, BioLegend), c-Kit (2B8, 1:400, BioLegend), CD45.1 (A20, 1:200, BioLegend), and CD45.2 (104, 1:200, Biolegend) were used for flow cytometric analysis. Flow cytometric analysis and cell sorting were conducted on BD FACS Aria III cell sorter (BD Biosciences).
Transplantation
Either 100 tdTomatohi or 5000 tdTomato− cells obtained from Hlf-tdTomato KI mice (Ly5.1/Ly5.2) were intravenously transplanted into lethally irradiated C57BL/6 (Ly5.2) mice (total 10 Gy) along with Ly5.1 whole bone marrow cells (2 × 105 cells) as competitor cells. Chimerism in the peripheral blood of the recipients was analyzed by flow cytometry at the indicated time points33. For Fig. 1c, the peripheral blood was obtained from the same set of mice.
Intravital tibial bone marrow imaging
Hlf-tdTomato KI adult and neonatal mice were anesthetized with isoflurane using an inhalational anesthesia system34,35,36. Anesthesia was maintained using 1.0–1.5% isoflurane at a flow rate of ~0.2 L/min air. Anesthetized mice were placed on heating pads and monitored to maintain body temperature. The skin around the tibia was cut and the tibia was fixed to reduce the artifacts caused by the heartbeat and breathing to image the tibial bone marrow. A coverslip was placed on the exposed tibia. Intravenous injection of Qtracker 655 (Invitrogen) was conducted by retro-orbital injection (in adult mice) or superficial temporal vein injection using a pulled glass capillary (in neonatal mice) immediately prior to imaging. Intravital imaging of the tibial bone marrow was performed using a customized multiphoton laser-scanning upright microscope (Leica, SP8MP) with a 25× water immersion objective having a numerical aperture of 0.95 (Leica) and fiber oscillators that deliver pulses at 1070 nm (Coherent, Fidelity-2) and 920 nm (Spark lasers, Alcor-920). Fluorescence was collected by the 25× objective and directed to two HyD detectors (Leica) and one photomultiplier tube. Three-dimensional images of the bone marrow with a field of view of ~600 μm × 600 μm (512 × 512 pixels) were acquired every 5 μm.
Image analysis to measure the velocity of tdTomato-positive cell movement
General image processing was performed with ImageJ software37. Image registration was performed with the ImageJ plugin Correct 3D Drift38 to correct the specimen drift in time-lapse images. The background noise was eliminated by masking the registered images with the binary tdTomato-positive cell images (mask images). Mask images were obtained from registered images with a 4–5 pixel-bandpass filtering, Otsu’s thresholding, and manual postprocessing. The masked time-lapse images of tdTomato-positive cells were used to measure the velocity of the cell movement via cell tracking using the ImageJ plugin TrackMate30.
Histology and confocal microscopy
Bones were fixed with 1% paraformaldehyde in phosphate buffer (PB) overnight at 4 °C and then incubated in 30% sucrose in PB for 1–2 days. Samples were embedded in O.C.T. Compound (Sakura Finetek), and then thick sections (more than 150 μm) of bone marrow were cut using a cryostat (Leica). Rabbit anti-DsRed antibody (for tdTomato) (632496, 1:500, Takara Bio Clontech), Alexa Fluor 488-conjugated anti-rabbit IgG (H + L) antibody (711-545-152, 1:1000, Jackson ImmunoResearch), PE-conjugated CD45.1 antibody (110708, 1:100, BioLegend), PE-conjugated CD45.2 antibody (109808, 1:100, BioLegend), Alexa Fluor 647-conjugated TER-119 antibody (116218, 1:100, BioLegend), and 4′,6-diamidino-2-phenylindole (DAPI) (10 μg/mL, Thermo Fisher Scientific) were used for histological staining. Bone sections were imaged using an SP8LS confocal microscope (Leica).
RNA-seq
For RNA-seq, 100 sorted HSCs were used to synthesize first-strand cDNA with PrimeScript RT reagent kit (TAKARA Bio Inc.) and not-so-random primers4,39. First-strand cDNA was treated with Klenow fragments (3′-5′ exo; New England Biolabs Inc.) and complement chains of not-so-random primers to synthesize the second-strand cDNA. The double-stranded cDNA was purified and an RNA-seq library was prepared and amplified using the Nextera XT DNA sample prep kit (Illumina Inc.). Prepared libraries were sequenced using Nextseq 500 (Illumina Inc.) and each obtained read was mapped to the reference sequence of mm10 using CLC genomic workbench, v11.0.0 (Qiagen). Expression levels were normalized and subjected to statistical analyses based on edgeR. Transcriptome data were subjected to GSEA using GSEA v4.0.3 software40. Gene sets were obtained from the Broad Institute database. Transcriptome data were repeatedly used for the analysis of the different gene sets.
Statistical and reproducibility
All results are presented as mean ± standard error. The significance of the differences was analyzed using a two-tailed Student’s t-test (Fig. 4d) or a two-tailed Mann–Whitney U-test (Fig. 5b and Supplementary Fig. 2b) using Microsoft Excel and Statistical Package for the Social Sciences. Kolmogorov-Smirnov test was used to calculate the normality. Hedges’ g or r was calculated to obtain the effect size. Hedges’ g was obtained by dividing the mean difference by the pooled standard deviation. The pooled standard deviation was calculated as the square root of {(n1 − 1)SD12 + (n2 − 1)SD22}/{(n1 − 1) + (n2 − 1)}, where n1 and n2 are the sample number of each group, and SD1 and SD2 are the standard deviations of each group. r was obtained by dividing z by the square root of N, where z is the test statistic obtained by the U-test and N is the sample size. All p values <0.05 were considered statistically significant. The required sample size was calculated by R3.6.341 using power.t.test function with the following arguments: sig.level = 0.05, power = 0.8, d = 1, and SD = 1 (d = 1, SD = 1 were used to set the effect size as 1). Note that, for intravital imaging experiments, the sample number is lower than the required sample size due to technical difficulty.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data were available upon reasonable request to the corresponding author (H.M.). RNA-seq data is available in the Gene Expression Omnibus repository (accession number: GSE16691).
References
Nakamura-Ishizu, A., Takizawa, H. & Suda, T. The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development 141, 4656–4666 (2014).
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Bowie, M. B. et al. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J. Clin. Invest. 116, 2808–2816 (2006).
Hashimoto, M. et al. Autophagy is dispensable for the maintenance of hematopoietic stem cells in neonates. Blood Adv. 5, 1594–1604 (2021).
Cheng, T. et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 (2000).
Li, Y. et al. Single-cell analysis of neonatal HSC ontogeny reveals gradual and uncoordinated transcriptional reprogramming that begins before birth. Cell Stem Cell 27, 732–747.e737 (2020).
Khan, J. A. et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351, 176–180 (2016).
Wang, L. D. & Wagers, A. J. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat. Rev. Mol. Cell Biol. 12, 643–655 (2011).
Christensen, J. L., Wright, D. E., Wagers, A. J. & Weissman, I. L. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2, E75 (2004).
Christodoulou, C. et al. Live-animal imaging of native haematopoietic stem and progenitor cells. Nature 578, 278–283 (2020).
Lo Celso, C. et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92–96 (2009).
Mazo, I. B. et al. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J. Exp. Med. 188, 465–474 (1998).
Rashidi, N. M. et al. In vivo time-lapse imaging shows diverse niche engagement by quiescent and naturally activated hematopoietic stem cells. Blood 124, 79–83 (2014).
Fujisaki, J. et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474, 216–219 (2011).
Kim, S., Lin, L., Brown, G. A. J., Hosaka, K. & Scott, E. W. Extended time-lapse in vivo imaging of tibia bone marrow to visualize dynamic hematopoietic stem cell engraftment. Leukemia 31, 1582–1592 (2017).
Köhler, A. et al. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood 114, 290–298 (2009).
Upadhaya, S. et al. Intravital imaging reveals motility of adult hematopoietic stem cells in the bone marrow niche. Cell Stem Cell 27, 336–345.e334 (2020).
Cao, X. et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc. Natl Acad. Sci. USA 108, 1609–1614 (2011).
Hooper, A. T. et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274 (2009).
Zhou, B. O. et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 19, 891–903 (2017).
Shimoto, M., Sugiyama, T. & Nagasawa, T. Numerous niches for hematopoietic stem cells remain empty during homeostasis. Blood 129, 2124–2131 (2017).
Acar, M. et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130 (2015).
Chan, C. K. et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494 (2009).
Lassailly, F., Foster, K., Lopez-Onieva, L., Currie, E. & Bonnet, D. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood 122, 1730–1740 (2013).
Yokomizo, T. et al. Hlf marks the developmental pathway for hematopoietic stem cells but not for erythro-myeloid progenitors. J. Exp. Med. 216, 1599–1614 (2019).
Giladi, A. et al. Single-cell characterization of haematopoietic progenitors and their trajectories in homeostasis and perturbed haematopoiesis. Nat. Cell Biol. 20, 836–846 (2018).
Qiu, J., Papatsenko, D., Niu, X., Schaniel, C. & Moore, K. Divisional history and hematopoietic stem cell function during homeostasis. Stem Cell Rep. 2, 473–490 (2014).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Ng, C. E. et al. A Runx1 intronic enhancer marks hemogenic endothelial cells and hematopoietic stem cells. Stem Cells 28, 1869–1881 (2010).
Tinevez, J. Y. et al. TrackMate: an open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017).
Coşkun, S. et al. Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep. 9, 581–590 (2014).
Bixel, M. G. et al. Flow dynamics and HSPC homing in bone marrow microvessels. Cell Rep. 18, 1804–1816 (2017).
Umemoto, T., Hashimoto, M., Matsumura, T., Nakamura-Ishizu, A. & Suda, T. Ca2+-mitochondria axis drives cell division in hematopoietic stem cells. J. Exp. Med. 215, 2097–2113 (2018).
Mizuno, H. et al. Patchwork-type spontaneous activity in neonatal barrel cortex layer 4 transmitted via thalamocortical projections. Cell Rep. 22, 123–135 (2018).
Mizuno, H. et al. NMDAR-regulated dynamics of layer 4 neuronal dendrites during thalamocortical reorganization in neonates. Neuron 82, 365–379 (2014).
Mizuno, H. et al. NMDA receptor enhances correlation of spontaneous activity in neonatal barrel cortex. J. Neurosci. 41, 1207–1217 (2021).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to imageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Parslow, A., Cardona, A. & Bryson-Richardson, R. J. Sample drift correction following 4D confocal time-lapse imaging. J. Vis. Exp. 51086 (2014).
Ozsolak, F. et al. Digital transcriptome profiling from attomole-level RNA samples. Genome Res. 20, 519–525 (2010).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Ihaka, R. & Gentleman, R. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5, 299–314 (1996).
Acknowledgements
We would like to thank Hiromi Mizuno for their assistance regarding experiments. This research was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology (Grants-in-Aid for Scientific Research JP20K06876 and 20K09774).
Author information
Authors and Affiliations
Contributions
Y.T. and H.M. designed the study and wrote the first draft of the manuscript. Y.T., T.H., and H.M. designed the computational analysis of in vivo imaging data. Y.T., T.H., T.Y., T.U., K.A., M.H., M.S., H.T., T.I., T.S., and H.M. performed experiments/analyzed and interpreted data. T.Y., T.U., and T.S. revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Eirini Trompouki and Eve Rogers. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takihara, Y., Higaki, T., Yokomizo, T. et al. Bone marrow imaging reveals the migration dynamics of neonatal hematopoietic stem cells. Commun Biol 5, 776 (2022). https://doi.org/10.1038/s42003-022-03733-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-022-03733-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.