Abstract
Appetitive and aversive learning are both key building blocks of adaptive behavior, yet knowledge regarding their differences is sparse. Using a capsaicin heat pain model in 36 healthy participants, this study directly compared the acquisition and extinction of conditioned stimuli (CS) predicting pain exacerbation and relief. Valence ratings show stronger acquisition during aversive compared to appetitive learning, but no differences in extinction. Skin conductance responses and contingency ratings confirmed these results. Findings were unrelated to individual differences in pain sensitivity or psychological factors. Our results support the notion of an evolutionarily hardwired preponderance to acquire aversive rather than appetitive cues as is protective for acute aversive states such as pain but may contribute to the development and maintenance of clinical conditions such as chronic pain, depression or anxiety disorders.
Similar content being viewed by others
Introduction
Successful navigation of our physical and psychological environment requires various skills—but arguably, learning from mistakes is amongst the most critical. If an encounter has led to pain in the past, animals and humans alike go to great lengths to avoid similar incidents in the future by learning to predict them from cues that signal upcoming pain. Experimental learning studies have extensively investigated this associative learning. Previously neutral stimuli of events that are repeatedly paired with unpleasant stimuli or pain (unconditioned stimulus, US) begin to function as predictive cues (conditioned stimulus, CS) and themselves become capable of triggering physiological and emotional responses to pain. However, over time the predictive value of the cue can change, and the cue might no longer be followed by pain. In this case, our representation of the association between cue and pain needs to be updated to represent the change in contingency. Although this extinction learning has been investigated in numerous studies, the principles guiding it are still subject to debate1. While early concepts assumed that extinction only requires erasure of previous learning, more recent accounts propose that it includes new learning that inhibits the originally formed associative learning (e.g., Pearce-Hall modell2).
Potential extinction and acquisition differences between appetitive and aversive, e.g., pain-related, CS–US associations might also be due to differences in their biological relevance. Although appetitive stimuli elicit approach behavior, aversive stimuli or events signal potential threats and trigger protective and avoidance behavior. Thus, a more conservative updating rule for aversive than for appetitive CS–US associations reduces the risk of underestimating the threat signaled by cues that once predicted harm, even when the chances of aversive consequences have become very small1. This behavior could be seen as a “better-safe-than-sorry” strategy3 that ensures that updating during extinction learning is delayed until a more conservative threshold for safe extinction has been reached. Overly fast acquisition and slow or incomplete extinction of aversive CS–US associations are assumed to contribute to the development and maintenance of several diseases including chronic pain4,5,6, although empirical evidence for this assumption has been inconsistent, so far7,8.
In order to test whether aversive and appetitive learning of predictive cues are guided by different learning rules, the two types of learning have to be compared directly within the same model and during both learning phases (i.e, acquisition and extinction). Experimentally induced tonic pain is an ideal model for this purpose as it allows to combine cues (CS) signaling aversive events (i.e., transient increases in pain) and appetitive events (i.e., transient relief from pain) within the same paradigm. To date, only a few studies have directly compared conditioned responses to cues predicting pain increase and pain relief, but their design either only included the acquisition phase9 or compared pain and reward in different sensory modalities (i.e., pain vs. food)10.
Here, we directly compared associative learning about pain and relief during both acquisition and extinction within the same sensory modality using a novel capsaicin-induced tonic heat pain model in healthy volunteers. Capsaicin increases the sensitivity to noxious and innocuous stimuli which means that safe, low-level heat stimuli can be used to induce lasting individually calibrated heat pain that resembles clinical pain but is easily modifiable through temperature manipulations10,11,12.
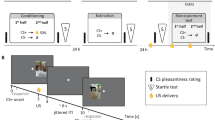
Tonic, moderate pain was induced for ~45 minutes in healthy volunteers by applying thermal stimulation to the forearm that had been pretreated with capsaicin. Temperatures were individually calibrated to induce moderate ongoing pain as well as phasic increases and decreases of pain. Geometrical figures were used as visual cues. During the acquisition phase, one cue (CSincrease) predicted pain exacerbation (USincrease), another cue (CSdecrease) predicted pain decrease (USdecrease), and a third cue (CSmedium) predicted no change in ongoing pain (USmedium). During the extinction phase, all cues were presented without changes in temperature (USmedium only) (see Fig. 1). Note that in contrast to previous studies that focused on the effect of conditioning on US perception13,14,15,16, our study investigates changes in CS perception and learning in terms of valence ratings as the main outcome measure. Further, contingency ratings of CS–US-associations and skin conductance responses (SCR) were recorded. Pain intensity and (un)pleasantness ratings of the US were only collected to validate task manipulations.
A pain intensity of about VAS 40 was induced by applying thermal heat stimuli to capsaicin-pretreated skin (tonic pain). An increase and decrease in temperature level led to pain exacerbation (VAS 80) and pain relief (VAS 0), respectively. The design consisted of habituation, acquisition, and extinction training. Assignment of geometric figures to experimental conditions was pseudo-randomized (example shown here).
Based on the assumption that aversive learning requires less evidence than appetitive learning, we hypothesized that CS predicting pain exacerbation is associated with enhanced differential learning (i.e., steeper acquisition slopes) and slower differential extinction slopes in valence ratings compared to CS predicting pain relief. In addition, we explored whether pain-related associative learning depended on individual pain-related cognitions17, including pain anxiety, pain catastrophizing, pain sensitivity and state and trait depression and stress measures, or changes in physiological responses to CS and US.
Our results show stronger acquisition during aversive compared to appetitive learning in terms of valence ratings, but no differences in extinction. These results support the notion of an evolutionarily hardwired preponderance to acquire aversive rather than appetitive cues as is protective for acute aversive states such as pain.
Results
US pain intensity and (un)pleasantness ratings
Our experimental pain model successfully induced a moderate level of tonic pain (VAS 40) and the intended transient increases and decreases in pain intensity following the CSincrease and CSdecrease (see Fig. 2). Differential perception of USincrease, USdecrease, and USmedium was confirmed by analysis of pain intensity and (un)pleasantness ratings. Analyses of pain intensity ratings acquired during training and acquisition revealed a significant main effect of US type. As intended, the USincrease was rated as significantly more painful than the USmedium (Δβ: 37.27 ± 3.25; t(83.83) = 11.48, p < 0.001, d = 2.51) and the USdecrease was rated as significantly less painful than the USmedium (Δβ: −35.83 ± 3.01; t(99.13) = −11.89, p < 0.001, d = −2.39). USmedium pain intensity ratings habituated slightly over time as indicated by a significant main effect for the factor phase with decreasing pain intensity ratings from acquisition to extinction training (β: −7.22 ± 3.13; t(175.00) = −2.31, p = 0.02, d = −0.35). Pain intensity ratings for the transient USincrease and USdecrease showed no significant change over time (all p > 0.05).
Pain intensity (a) and pain (un)pleasantness (b) ratings during all experimental phases for all US types on 0–100 VAS (A) and −50–50 VAS (B) in mean ± standard error of the mean of raw values. Displayed are ratings for the USincrease and USdecrease during training (train) and acquisition (acq) and ratings for the USmedium during training, acquisition, and extinction (ext). Single data points are displayed in gray. Data are provided for N = 36 subjects.
The three US types were also rated differently with respect to (un)pleasantness as indicated by a significant main effect for the factor US type. USdecrease was rated significantly more pleasant than USmedium (Δβ: −34.15 ± 3.34; t(79.38) = −10.21, p < 0.001, d = −2.29; see Fig. 2) and USincrease was rated significantly more unpleasant than USmedium (Δβ: 24.57 ± 2.79; t(120.07) = 8.81, p < 0.001, d = 1.61). Importantly, (un)pleasantness ratings did not change over time for either US type which suggests that changes in valence ratings are not due to increases or decreases in US (un)pleasantness. To formally test a potential influence of US intensity on emotional learning, changes in individual US intensity ratings over time were added as covariates of no interest when analyzing CS valence ratings. None of the tested covariates improved model fit for either pain intensity or (un)pleasantness ratings.
See Supplementary Figure 1 for the development of US ratings over the course of the experimental phases.
Calibrated temperatures, ratings, and questionnaire results can be seen in Supplementary Table 1.
Valence ratings of the conditioned stimuli
Participants showed successful differential learning, i.e., an increase in negative valence for CSincrease and an increase in positive valence for CSdecrease during acquisition training as well as successful extinction, i.e., a decrease in negative valence for CSincrease and a decrease in positive valence ratings for CSdecrease during extinction training. Individual CS-specific valence ratings and differential data in valence ratings for CSincrease and CSdecrease compared with CSmedium are shown in Fig. 3.
a Valence ratings (raw value) during the habituation (Hab), acquisition (Acq 1–Acq 4), and extinction phases (Ext 1–Ext3).b Differential valence ratings (raw value) of CSincrease/CSdecrease relative to CSmedium (i.e., |(CSmedium−CSdecrease|) during the habituation (Hab), acquisition (Acq 1−Acq 4), and extinction phases (Ext 1−Ext3). An absolute difference of 0 (solid line) indicates equal valence ratings for CSincrease/CSdecrease and CSmedium. Ratings are given as means ± standard error of the mean. Single data points in transparent colors. Dashed lines separate the phases. Data are provided for N = 36 subjects.
Acquisition training. Valence ratings for CSmedium did not significantly change over the course of the acquisition phase (β: −0.24 ± 0.66; t(295.05) = −0.37, p = 0.71, d = −0.04) (Fig. 3a). Analyses of CS-specific valence ratings of acquisition training revealed a significant time x CS type interaction. Valence ratings for CSincrease showed an increase in negative valence over the course of the acquisition training relative to the CSmedium (Δβ: 9.39 ± 0.91; t(405.97) = 10.33, p < 0.001, d = 0.67). Valence ratings of CSdecrease became more positive over time compared to CSmedium ratings (Δβ: −3.19 ± 0.91; t(405.90) = −3.53, p < 0.001, d = −0.35).
The comparison of CSincrease and CSdecrease relative to CSmedium (i.e., (CSincrease−CSmedium) and (CSmedium−CSdecrease)) showed larger changes in valence ratings for CSincrease than CSdecrease during acquisition training (Δβ: 2.91 ± 1.14; t(224.80) = 2.56, p = 0.01, d = 0.34). To account for potential intra- and interindividual variability in absolute pain intensity and (un)pleasantness differences, we included those ratings as covariates in our analysis (calculated as (USincrease−USmedium) and (USmedium−USdecrease)). This neither revealed any significant interactions (all p > 0.05) nor did it improve model fit, which suggests that our results were not driven by differences in pain perception between USincrease and USmedium vs. USdecrease and USmedium. Similarly, CS valence learning was not significantly influenced by US-induced changes in electrodermal activity as the inclusion of differences in SCR amplitude (calculated as (USincrease−USmedium) and (USdecrease−USmedium)) did not improve model fit. Increased learning from USincrease compared to USdecrease was not related to any of the psychological variables (all p > 0.05).
Extinction training. As for the acquisition training, CS-specific valence ratings obtained during the extinction training showed a significant time x CS type interaction. CSmedium valence ratings did not significantly change over the course of the extinction training (β: 0.71 ± 0.79; t(304.75) = 0.90, p = 0.37, d = 0.10), while relative to CSmedium, the valence of CSincrease became less negative represented in an absolut decrease in numerical rating (Δβ: −6.28 ± 1.13; t(313.88) = −5.57, p < 0.001, d = −0.63), whereas the CSdecrease valence ratings became significantly less positive as indicated in an absolute increase in numerical rating (Δβ: 4.66 ± 1.12; t(313.04) = 4.16, p < 0.001, d = 0.47).
The direct comparison of CSincrease and CSdecrease (relative to CSmedium) revealed no significant differences in differential extinction learning between both CS+ (Δβ: 1.51 ± 1.42; t(171.14) = 1.06, p = 0.29, d = 0.16).
Although there was no difference in the pace of extinction learning between both CS+ (i.e., CSincrease and CSdecrease), we performed an explorative analysis showing incomplete extinction for the CSincrease only, as indicated by the difference between the last extinction rating and the valence rating during habituation prior to acquisition training. Relative to CSmedium, valence ratings for the CSincrease (Δβ: 1.41 ± 0.36; t(172.54) = 3.95, p < 0.001, d = 0.46) but not for the CSdecrease (Δβ: 0.29 ± 0.35; t(171.31) = 0.82, p = 0.41, d = 0.12) were significantly higher at the end of extinction training than during habituation.
None of the covariates improved model fit indicating that extinction learning was not significantly influenced by psychological traits.
Contingency ratings
Contingency ratings are displayed in Fig. 4.
Analyses revealed a significant decrease in contingency ratings from acquisition to extinction for CSincrease (β: −54.00 ± 6.09; t(175.00) = −8.87, p < 0.001, d = −1.34) and CSdecrease (β: −46.28 ± 6.09; t(175.00) = −7.60, p < 0.001, d = −1.15), but no significant change for the CSmedium (β: 5.61 ± 6.09; t(175.00) = 0.92, p = 0.36, d = 0.14). We also found a significant main effect of CS type. Across phases, both CSincrease and CSdecrease differed significantly from CSmedium (CSincrease Δβ: 71.06 ± 6.09; t(175.00) = 11.68, p < 0.001, d = 1.77; CSdecrease Δβ: 54.78 ± 6.09; t(175.00) = 9.00, p < 0.001, d = 1.36) and CSincrease yielded higher ratings than CSdecrease (Δβ: 16.28 ± 6.09; t(175.00) = 2.68, p = 0.008, d = 0.40). However, there was no significant interaction between the factors CS type and phase for the CSincrease and the CSdecrease, indicating that changes in contingency ratings from acquisition to extinction training did not differ between both CS+ (Δβ: −7.72 ± 8.61; t(175.00) = −0.90, p = 0.37, d = −0.14). Including potential covariates did not improve model fit indicating that contingency awareness was not significantly influenced by psychological traits.
Skin conductance responses
SCR recorded during trials without valence ratings were pooled, resulting in four pooled SCRs for acquisition training and three pooled SCRs for extinction training. The factor time was used as an indicator of change within each experimental phase. SCR amplitudes are shown in Figs. 5 and 6.
a SCR amplitudes for the CS during acquisition and extinction training. b SCR amplitudes to the US for the entire acquisition phase. SCR is given in log-transformed means ± standard error of the mean using the natural logarithm. Single data points in transparent colors. Data provided for n = 31 subjects.
Differential SCR amplitudes for the CS during acquisition and extinction training between CSincrease/CSdecrease and CSmedium (e.g., (CSmedium−CSdecrease). Ratings are given in log-transformed means ± standard error of the mean using the natural logarithm. Single data points in transparent colors. Data are provided for n = 30 subjects.
Unconditioned stimuli. During acquisition training, USincrease yielded higher SCR amplitudes than USdecrease (Δβ: 0.17 ± 0.03; t(30.63) = 5.51, p < 0.001, d = 1.99).
Conditioned stimuli. CS-specific SCRs collected during acquisition training showed a significant main effect of CS type. Consistent with results from the valence ratings, this analysis revealed overall higher SCR amplitudes for CSincrease than CSdecrease (Δβ: 0.06 ± 0.02; t(569.0) = 3.67, p < 0.001, d = 0.31). Differential effects relative to CSmedium (i.e., SCR CSincrease−SCR CSmedium and SCR CSdecrease−SCR CSmedium) did not differ significantly between both CS+ (Δβ: 0.02 ± 0.02; t(569.21) = 1.20, p = 0.23, d = 0.10). There was no effect of time indicating no changes in SCR amplitudes over the course of the acquisition training for any of the CS types (all p > 0.2).
For extinction training, we found a significant effect of CS type with higher SCR amplitudes for CSincrease than CSdecrease (Δβ: 0.12 ± 0.05; t(380.21) = 2.70, p = 0.008, d = 0.27). Moreover, the interaction with the factor time indicated a stronger decrease in SCR amplitude for CSincrease compared to CSdecrease (Δβ: −0.05 ± 0.02; t(378.84) = −2.56, p = 0.01, d = −0.27) but individual SCR related to CSdecrease and the CSmedium did not significantly change over time (all p > 0.1). There were no significant effects when comparing differential learning of both CS+ (all p > 0.3).
Discussion
This study used a capsaicin-heat pain model in healthy volunteers to directly compare aversive and appetitive conditioning during ongoing pain, focusing on both acquisition and extinction of CS–US associations. We showed enhanced acquisition learning during pain exacerbation compared to pain relief (i.e., steeper slopes in valence ratings for CSincrease versus CSdecrease). Importantly, these findings were not explained by interindividual differences in perceived pain intensity or physiological responses (SCR) to cues signaling pain or pain relief. By contrast, no differences between extinction slopes were observed although extinction of aversive learning did not return to baseline level, while valence of relief cues was comparable to baseline values at the end of the extinction phase. These results are corroborated by contingency awareness ratings and physiological responses to the CS. Together they underscore the higher significance of pain learning during acquisition, while a different, valence-independent process may be involved in extinction learning. These results are discussed within a broader context of appetitive versus aversive learning.
Previous studies have shown successful acquisition of both appetitive and aversive CS–US associations within various domains, including pain and pain relief9,10,18,19, food20, and odors21. These studies were unable to draw conclusions on the relative significance of pain compared to relief learning because they either investigated only pain learning or did not directly compare pain and relief learning within the same experimental model or learning phase. Our results substantially extend these findings by directly comparing the slopes of pain and relief learning within the same domain, while controlling for physiological responses to the CS and US intensity ratings. The observation that young healthy volunteers show greater acquisition of CSincrease compared to CSdecrease is in line with our hypothesis that learning from cues that predict an increase in pain is more important than predicting pain relief. Such shifts have so far mainly been studied in other, more general models of appetitive and aversive learning such as punishment and reward learning22,23,24,25,26, and have shown to be sensitive to various manipulations including pharmacological challenges (e.g., dopamine23,27), stress28,29,30,31, and inflammation32, where the latter two were associated with increased aversive compared to appetitive learning.
While the acquisition was stronger when participants were presented with the CSincrease than the CSdecrease, learning from the two cues did not differ during extinction training. Previous studies have so far mainly focused on the extinction of aversive CS and found evidence for a reoccurrence of previously extinguished conditioned responses in the form of spontaneous recovery, renewal, or reinstatement1. Although speculative at this point, this fragility of extinction could be interpreted as the result of an adaptive and evolutionarily advantageous ‘better-safe-than-sorry’ strategy as the high threshold for updating aversive CS associations should prevent the potentially costly mistake that a previously aversive predictive cue is now deemed safe when in fact it is not. However, if this was true, extinction slopes should reflect the differential pattern we see in acquisition slopes (i.e., faster extinction for CSdecrease than CSincrease) which is not supported by our valence ratings showing any significant differences in extinction slopes. More recent accounts of extinction learning claim that extinction is different from acquisition, as it involves interference from previously acquired associations1. In line with this view, we found the biological relevance of an aversive stimulus being reflected in the stronger acquisition of negative than of positive outcomes (pain increase vs pain relief). Instead, extinction may involve a different process that is less dependent on the biological relevance of the US, at least in healthy pain-free volunteers. Of note, valence ratings for the CSincrease were still significantly higher than baseline ratings at the end of extinction training, indicating incomplete extinction for CSincrease after 12 extinction trials. In contrast, the same number of extinction trials led to complete extinction in the case of the CSdecrease. However, the difference might also be explained by the higher negative valence of the CSincrease at the end of acquisition training. Even with the same extinction slope, a return to baseline would simply require more extinction trials. Hence, even though our data only indicate partial extinction for CSincrease, these results can not be interpreted as conclusive evidence for differential extinction for CSincrease and CSdecrease.
Our results showed that enhanced aversive compared to appetitive learning in healthy individuals was not linked to individual differences in trait anxiety or depression. This finding further supports our interpretation that the difference in learning might be adaptive rather than reflective of the excessive weighting of negative information as for instance seen in depression. While such a bias may constitute an evolutionary advantage in healthy individuals, especially in an acute stressful or potentially dangerous situation33, excessive weighting and anticipation of negative outcomes could become maladaptive and promote the development of pathological avoidance behaviors or psychopathologies like chronic pain, anxiety, and depression. Thus, chronic pain and pathological aversive states such as depression may be seen as the negative by-product of the strategy to prioritize learning from and engaging in acute aversive states.
Taken together, the current study is the first to directly compare learning and extinction of aversive and appetitive CS within the same experimental paradigm and demonstrates increased aversive versus appetitive learning that was independent of individual differences in US intensity ratings, trait anxiety, or depression. We propose that stronger learning from a cue that signaled an increase in pain (compared to one that signaled pain relief) may be reflective of a ‘better-safe-than-sorry’ strategy which ensures that aversive experiences are only no longer anticipated when it is safe to do so. Extinction, on the other hand, seems to be less dependent on the relevance of the US. The observed differences strongly indicate that extinction is more than a mirror process of acquisition but is governed by its very own learning rules.
Methods
Participants
In all, 43 healthy volunteers were recruited through local advertisements. Exclusion criteria were age <18 or >65 years, acute or chronic pain or other diseases including psychiatric disorders (all assessed based on self-report, clinically relevant levels of depression or anxiety (i.e., ADS-K score >18; Hautzinger et al., 2012), regular medication intake (except thyroid medication, allergy medication, occasional use of over-the-counter analgesics), body mass index >30 or <18, left-handedness, pregnancy or breastfeeding, known allergy to capsaicin and acute sunburn or other visible signs of dermatological abnormalities on the volar forearm. Only female subjects using hormonal contraceptives were included in the study. Participants were informed that the purpose of the study was to investigate visual perception and processing during noxious thermal stimulation. The study was approved by the local Ethics Committee (16-7248-BO) of the Medical Faculty, University Duisburg-Essen. All participants gave written informed consent and received monetary compensation for taking part in the study. Participants were free to withdraw from the study at any time.
Data of n = 7 participants were discarded from data analysis due to technical difficulties during data acquisition (n = 2) or perception of noxious stimuli not reaching a sufficiently painful level during calibration (n = 5). Behavioral data of the remaining 36 right-handed participants (19 female, age M ± SD 25.31 ± 4.29 years) were included in the analyses.
Differential conditioning paradigm
The study used a classical conditioning paradigm with geometric figures as conditioned stimuli (CS) and contact heat as unconditioned stimuli (US). It was divided into three experimental phases (see Fig. 1); habituation (CS presentation alone), acquisition training (CS presentation with 75% reinforcement rate), and extinction training (CS presentation without reinforcement). Temperature levels for moderate pain, pain increase, and pain decrease were calibrated individually for each participant prior to the actual experiment and reassessed during a training session (see below for details).
A square, rectangle, and rhombus served as CS to predict either pain exacerbation (CSincrease), pain relief (CSdecrease), or no change in tonic pain of moderate-intensity (CSmedium). Allocation of the geometric shapes to the three conditions was randomized across participants. CS was presented in blue color (RBG code: 142, 180, 227) on a black background (square: visual angle 4.99° × 4.99°, rectangle: visual angle 8.3° × 3.14°, rhombus: visual angle 7.38° × 5.36°) on a computer screen. Unconditioned stimuli consisted of a phasic increase (USincrease), decrease (USdecrease), or no intensity change of tonic pain (USmedium). Tonic pain was induced by applying individually calibrated thermal stimulation (Model ATS, Pathway System, Medoc, Israel, http://www.medoc-web.com) to the site on the volar forearm that had been pretreated with capsaicin cream (1%, 8-methyl-Nvanillyl-6-nonenamide, 98%, Sigma, diluted in 5% ethanol-KY jelly)9,18,34. Capsaicin is the active ingredient of chili peppers that binds to vanilloid receptors (TRPV1) and increases sensitivity to thermal stimulation9,18. Following the application of capsaicin cream, a phasic increase and decrease of pain can be achieved by applying different levels of contact heat via a thermal stimulation device.
In all experimental phases, CS was shown on the computer screen for 9 s, followed by a black screen for 10.5 s. US were presented for 8 s, starting during the last 1.5 s of CS presentation and lasting 6.5 s into the display of a black screen. The inter-trial-interval ranged from 6 and 11 s. CS were presented in a pseudo-randomized order with no more than three consecutive presentations of the same CS and CS conditions were equally distributed within the first and second half of acquisition and extinction training. During acquisition training, the first and last CS of each type were always reinforced. For training and acquisition, six different predefined randomization protocols were used.
Presentation of visual and thermal stimuli and recording of the behavioral data were controlled using the software Presentation (www.neurobs.com).
Behavioral outcome measures
To assess the temporal dynamics of acquisition and extinction of CS–US associations, participants were asked to provide valence ratings for each CS type throughout all experimental phases on a Visual Analog Scale, VAS (VAS: “How do you perceive this geometric figure?” -50=very pleasant, 0 = neutral, 50 = very unpleasant), which was displayed during the first 7 s of every fourth CS presentation of the same CS type (see Supplementary Material, Examples of VAS scales). To assess contingency awareness, participants were given 15 s at the end of acquisition and extinction training to rate how often each CS had been followed by a change in temperature on a VAS with anchors “100% cooling”, “no change”, and “100% heating”.
To test whether USincrease, USdecrease, and USmedium were perceived differently, participants provided pain intensity ratings (VAS 0–100 with anchors “0 = not painful at all” and “100 = unbearably painful”) and pain (un)pleasantness ratings (VAS 0–100 with anchors “−50 = very pleasant“, “0 = neutral” and “−50 = very unpleasant”). These VAS scales were presented for 4 s after the end of every fourth US. For USincrease and USdecrease, participants provided one pain intensity and (un)pleasantness rating during the training phase following calibration and three ratings during acquisition training, respectively. For USmedium, participants rated pain intensity and (un)pleasantness once during the training phase, three times during acquisition, and five times during extinction training (see Supplementary Table 2 for details).
Prior to the experiment, participants also rated their current arousal level (“How tense do you feel at the moment?”, anchors: “not tense at all”–“extremely tense”) and pain-related fear (“How fearful are you about the upcoming pain stimulation?”, anchors: “not fearful at all”–“extremely fearful”) using a VAS. All VAS cursor positions had a random start position between 25 and 75.
Skin conductance responses
To track changes in sympathetic arousal in response to CS and US, skin conductance was continuously recorded in all experimental phases using a BIOPAC MP150 system with the software AcqKnowledge 4.2 (BIOPAC Systems Inc). We used a bipolar recording with two disposable Ag/AgCl electrodes (0.8 cm diameter) and a conductive electrode cream (SYNAPSE®; Kustomer Kinetics). The electrodes were attached to the thenar and hypothenar eminences of the left hand. The sampling rate was set to 2 kHz and data were stored locally as text files for offline analysis.
Study procedures
The capsaicin cream was applied to a small area of 3 × 3 cm on the volar forearm using a cotton swab and covered with a patch. After 45 minutes, the cream was removed with a dry tissue and the thermode was attached to the capsaicin-pretreated site.
Experimental phases
Temperature calibration and training
In order to be able to investigate pain-related learning under realistic conditions but combined with the advantages of a controlled experimental setting, we developed an experimental model of tonic pain that would allow for repeated, deliberate variations in pain intensity. This required a calibration procedure that takes into account habituation and sensitization processes of pain that commonly occur in tonic pain models35,36,37. The following protocol is the result of extensive piloting to meet this requirement.
Temperature calibration was carried out individually for each participant to determine three temperature levels that were perceived as ‘very painful’ (VAS pain = 80), ‘moderately painful’ (VAS pain = 40), and ‘not painful’ (VAS pain = 0). To this end, a staircase procedure was applied twice, in which continuous heat was applied, starting at 28 °C and increasing in steps of 2 °C until the participant indicated a pain intensity level >70 on the VAS pain. A moderately painful temperature of VAS pain 50 was then used to determine the range for the next procedure. In this procedure, ten different temperature levels (−1.5 °C to +3.0 °C) were applied twice in a semi-randomized order. The temperature level was kept constant for 8 s before it returned to a non-painful baseline intensity of 26 °C. Using a linear regression, temperature levels corresponding to VAS60 and 80 were determined and used for tonic pain (USmedium) and pain exacerbation (USincrease), respectively. The temperature level for USdecrease was calculated as the temperature determined for tonic pain minus 15 °C (minimum 20 °C). The three temperature levels were then presented three times in a semi-randomized order to reassess pain and (un)pleasantness ratings (training session). The total time for calibration was ~20 min.
Habituation
After temperature calibration, contact heat at the moderate-intensity level of USmedium was continuously applied for the remainder of the experiment (~40 min). In addition, each CS was presented three times and participants rated their valence on a VAS (VAS: 3 ratings per CS type, nine ratings total).
Acquisition training
CSincrease and CSdecrease (16 CS each) were contingently paired with USincrease and USdecrease, respectively (75% reinforcement rate; = 12 reinforced CS of each type, 24 reinforced CS total), while the CSmedium (16 CS) was not paired with changes in temperature (USmedium). CS valence ratings were assessed on every fourth presentation of each CS (VAS, four ratings per CS type). US ratings were assessed on every third reinforced CSincrease and CSdecrease trial, resp. or every fourth CSmedium trial (VAS pain and VAS pleas: three ratings per US type).
Extinction training
The three CS types were presented without changes in temperature (12 CS each, 36 CS total) in order to extinguish the acquired CS–US associations. CS ratings were assessed on every fourth CS presentation (three ratings per CS type). USmedium pain intensity/or (un)pleasantness ratings were assessed five times.
Note that participants were informed about potential CS–US associations without giving further information, e.g., about different experimental phases, actual CS–US contingencies, or the absence of temperature modulation during extinction training.
Psychological questionnaires
All participants completed the German versions of the following psychological questionnaires: (1) the Pain Anxiety Symptom Scale: PASS-D38,39; (2) Pain Catastrophizing Scale: PCS40,41; (3) Pain Sensitivity Questionnaire: PSQ-2042; (4) Center for Epidemiological Studies Depression Scale43,44; (5) State Trait Anxiety Depression Inventory: STADI45; and (6) Depression Anxiety Stress Scales: DASS46.
Statistical analyses
The software R47 was used for all behavioral analyses. Linear mixed model analyses were performed on all outcome measures using the lme4 package48. Please see Supplementary Table 3 for details on model calculation and comparisons. All questionnaires were analyzed following their respective manual. Results with p < 0.05 are considered statistically significant.
Pain intensity and (un)pleasantness ratings. Analyses were performed to investigate differences in pain intensity and (un)pleasantness ratings between CS types and experimental phases. The first model included USmedium ratings from all experimental phases, whereas a second model included USmedium, USincrease, and USdecrease ratings from only the training phase and acquisition training. The calculated models contained the factors phase and US type (only for the model with USincrease and USdecrease) as fixed effects and random intercept for the subjects to allow for subject-specific variation. The models were estimated according to the restricted maximum likelihood (REML) approach. Potential exploratory covariates were included in the models to account for their modulating influences. This comprised gender, age, pain-related fear, anxiety and depression ratings, and pain catastrophizing.
CS valence ratings. Analyses were performed to test for changes in CS valence ratings over time and differences between conditions. Time × CS type effects were assessed in two different analyses. The first analysis assessed the direction of learning (i.e., CS valence increased or decreased over time) and included individual CS-specific valence ratings. The second analysis assessed differential learning of cues predicting pain exacerbation and pain relief (CSincrease and CSdecrease, respectively). To this end, absolute differences were calculated between the CSincrease and the CSmedium and the CSdecrease and the CSmediate for each time point (e.g., |(CSincrease rating1 - CSmedium rating1)|), respectively. Analysis steps for both model versions were identical and are described below.
Separate models were calculated for acquisition and extinction. The last valence rating of habituation was included as a baseline rating prior to CS−US coupling in the analysis of acquisition training as the first valence rating in acquisition training was only provided after three CS−US pairings. Likewise, the last rating of acquisition training was included as a baseline in the extinction training model. To account for differences between CS types and changes over time, the factors CS type and time were included as fixed effects into the models. Random intercepts and slopes for subjects were included to account for subject-specific variation. The factor time was included as a continuous factor in order to account for increases and decreases over the course of the experimental phases. We tested whether the model fit improved when allowing variation for the factors CS type and time by adding random slopes for these factors. All models on CS valence ratings were estimated according to the REML approach. The best model was selected based on Akaike information criterion (AIC). The models including subject-specific random slopes and random effects of the factors time and CS type predicted the data best as compared to models without random slopes (acquisition training: ΔAIC = −103.1, p < 0.001; extinction training: ΔAIC = −123.0, p < 0.001, see Supplementary Methods, Supplementary Table 3 for details of model comparison).
As we found significant differences in differential pain and relief learning during acquisition training (see Results section for details), we also accounted for a potential contribution of intra-individual differences in US pain intensity and (un)pleasantness. To this end, we calculated the difference in pain intensity and (un)pleasantness ratings between USincrease−USmedium and USdecrease−USmedium and included those as covariates in the model. We also tested whether the US-induced SCR during acquisition training (i.e., SCR amplitude changes induced by pain increase and pain decrease) correlated with CS valence ratings by including the difference of SCR between USincrease−USmedium and USdecrease−USmedium in the model. Potential covariates (i.e., gender, age, pain-related fear, anxiety and depression ratings, and pain catastrophizing) were included to test for their modulatory influence.
US-CS contingency ratings. Analyses were performed to test for differences in US-CS contingency ratings between phases and CS types. The model contained the factors phase (i.e. acquisition and extinction) and CS type as fixed effects and random intercepts and slopes for subjects. Again, we tested whether allowing variation for the factors CS type and phase by adding random slopes for these factors, improved model fit. The model was estimated according to the REML approach. AIC was used to identify the model that fitted the data best. According to the AIC, the model with random slopes for subjects and without random effects for the factor phase best predicted the data (compared to random slopes excluded: ΔAIC = −6.9, p = 0.004). Potential covariates (see above) were included in the model to account for their modulating influences.
Skin conductance responses. Due to technical difficulties during data acquisition in n = 5 participants, the analysis of SCR data is based on 31 participants. The software R was used for processing and analysis of the recorded skin conductance data. First, data were down-sampled to 20 Hz and smoothed with a low pass filter with a cutoff frequency of 2 Hz. Subsequently, local minima and maxima were automatically detected in the skin conductance trace and the amplitude of stimulus-related SCR was calculated by subtracting the local minimum at the onset of the first SCR following stimulus onset from the subsequent peak. For the CS, the response window for the SCR onset was set to 1 and 4 s after CS stimulus onset49. For the US, the response window was set to 1–8 s after US stimulus onset, which corresponds to US duration. This larger window was chosen to account for the rise and fall time of the contact heat and the total US duration. The minimum amplitude criterion was set to 0.01 μS such that smaller responses were scored as 0 µS. These values were log-transformed using the natural logarithm to reduce the skew of the amplitude distribution50. In order to avoid contamination of CS and US-induced SCRs with arm and hand movement during VAS ratings, we excluded all rating trials from the SCR analysis.
Linear mixed model analyses were performed on SCR amplitudes in each experimental phase separately to test for differences between CS and US types.
Analogously to the valence analyses, we assessed SCR changes (i.e., SCR CSincrease or CSdecrease over time) and included individual CS-specific SCR data in the model. A second analysis assessed differences in SCR amplitudes between CSincrease and CSdecrease relative to the CSmedium. To this end, absolute differences were calculated between SCR amplitudes induced by the CSincrease vs. CSdecrease (both relative to CSmedium).
The factor CS type or US type, respectively, was included in the models as a fixed effect and random intercepts and slopes for subjects were included in the model. For the model investigating CS-induced SCR, the factor time was included as a fixed effect. To this end, SCR amplitudes between trials with valence ratings were pooled resulting in four blocks of pooled SCR amplitudes for acquisition training and three blocks for extinction training. Further, it was tested whether allowing variation for the factors CS type/US type by adding random slopes for these factors improved model fit. The models were estimated according to the REML approach. The best model fit was based on AIC.
For the analyses of CS-induced SCRs, the models without the random factor CS type best predicted the data as compared to the model with random slopes for CS type (acquisition training: ΔAIC = −20.80, p < 0.001; extinction training: ΔAIC = −5.1, p = 0.43). For the analysis of US-induced SCRs, the model with the random factor US type was not predicted due to the limited number of observations. Please see supplementary material for details in model comparisons.
Statistics and reproducibility
Statistical analyses were conducted using the software R as described above. All information on experimental details needed on the reproducibility of the experiment is given in this manuscript and the supplementary material. Sample sizes are given in the section “Participants” and in the figure legends. Analyzed data does not include any replicates.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Behavioral and skin conductance data is provided in https://osf.io/gnk65/?view_only=dcbb22550e684a14bb3a31490ed0c6ae51. Further information on data will be available upon request to the corresponding author (KS). Figures 2–5 contain raw data.
References
Dunsmoor, J. E., Niv, Y., Daw, N. & Phelps, E. A. Rethinking extinction. Neuron 88, 47–63 (2015).
Pearce, J. M. & Hall, G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol. Rev. 87, 532–552 (1980).
Solomon, R. L. & Wynne, L. C. Traumatic avoidance learning: the principles of anxiety conservation and partial irreversibility. Psychol. Rev. 61, 353–385 (1954).
Schneider, C., Palomba, D. & Flor, H. Pavlovian conditioning of muscular responses in chronic pain patients: central and peripheral correlates. Pain 112, 239–247 (2004).
Icenhour, A. et al. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol. Motil. 27, 114–127 (2015).
Flor, H. New developments in the understanding and management of persistent pain. Curr. Opin. Psychiatry 25, 109–113 (2012).
Harvie, D. S., Moseley, G. L., Hillier, S. L. & Meulders, A. Classical conditioning differences associated with chronic pain: a systematic review. J. Pain. 18, 889–898 (2017).
Meulders, A. Fear in the context of pain: Lessons learned from 100 years of fear conditioning research. Behav. Res. Ther. 131, 103635 (2020).
Seymour, B. et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat. Neurosci. 8, 1234–1240 (2005).
Andreatta, M. & Pauli, P. Appetitive vs. Aversive conditioning in humans. Front Behav. Neurosci. 9, 128 (2015).
Petersen, K. L. & Rowbotham, M. C. A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport 10, 1511–1516 (1999).
Leknes, S., Brooks, J. C., Wiech, K. & Tracey, I. Pain relief as an opponent process: a psychophysical investigation. Eur. J. Neurosci. 28, 794–801 (2008).
Colloca, L., Sigaudo, M. & Benedetti, F. The role of learning in nocebo and placebo effects. Pain 136, 211–218 (2008).
Kessner, S., Sprenger, C., Wrobel, N., Wiech, K. & Bingel, U. Effect of oxytocin on placebo analgesia: a randomized study. JAMA 310, 1733–1735 (2013).
Wrobel, N., Wiech, K., Forkmann, K., Ritter, C. & Bingel, U. Haloperidol blocks dorsal striatum activity but not analgesia in a placebo paradigm. Cortex 57C, 60–73 (2014).
Zunhammer, M., Gerardi, M. & Bingel, U. The effect of dopamine on conditioned placebo analgesia in healthy individuals: a double-blind randomized trial. Psychopharmacology 235, 2587–2595 (2018).
Nees, F. & Becker, S. Psychological processes in chronic pain: influences of reward and fear learning as key mechanisms - behavioral evidence, neural circuits, and maladaptive changes. Neuroscience 387, 72–84 (2018).
Hindi Attar, C., Finckh, B. & Buchel, C. The influence of serotonin on fear learning. PLoS One 7, e42397 (2012).
Andreatta, M. & Pauli, P. Learning mechanisms underlying threat absence and threat relief: influences of trait anxiety. Neurobiol. Learn Mem. 145, 105–113 (2017).
Lovibond, P. F., Satkunarajah, M. & Colagiuri, B. Extinction can reduce the impact of reward cues on reward seeking behaviour. Behav. Ther. 46, 432–438 (2015).
Gottfried, J. A., O’Doherty, J. & Dolan, R. J. Appetative and aversive olfactory learning in humans fMRI. J. Neurosci. 22, 10829–10837 (2002).
van der Schaaf, M. E., Fallon, S. J., Ter Huurne, N., Buitelaar, J. & Cools, R. Working memory capacity predicts effects of methylphenidate on reversal learning. Neuropsychopharmacology 38, 2011–2018 (2013).
van der Schaaf, M. E. et al. Establishing the dopamine dependency of human striatal signals during reward and punishment reversal learning. Cereb. Cortex 24, 633–642 (2014).
Frank, M. J., Seeberger, L. C. & O’Reilly, R. C. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science 306, 1940–1943 (2004).
Pessiglione, M., Seymour, B., Flandin, G., Dolan, R. J. & Frith, C. D. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442, 1042–1045 (2006).
Westbrook, A. et al. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science 367, 1362–1366 (2020).
Wood, P. B. Mesolimbic dopaminergic mechanisms and pain control. Pain 120, 230–234 (2006).
Robinson, O. J., Overstreet, C., Charney, D. R., Vytal, K. & Grillon, C. Stress increases aversive prediction error signal in the ventral striatum. Proc. Natl Acad. Sci. USA 110, 4129–4133 (2013).
Bogdan, R. & Pizzagalli, D. A. Acute stress reduces reward responsiveness: implications for depression. Biol. Psychiatry 60, 1147–1154 (2006).
Berghorst, L. H., Bogdan, R., Frank, M. J. & Pizzagalli, D. A. Acute stress selectively reduces reward sensitivity. Front. Hum. Neurosci. 7, 133 (2013).
Elsenbruch, S. & Wolf, O. T. Could stress contribute to pain-related fear in chronic pain? Front. Behav. Neurosci. 9, 340 (2015).
Harrison, N. A. et al. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol. Psychiatry 80, 73–81 (2016).
Ruggeri, K. et al. Replicating patterns of prospect theory for decision under risk. Nat. Hum. Behav. 4, 622–633 (2020).
Price, R. C. et al. Characterization of a novel capsaicin/heat ongoing pain model. Eur. J. Pain. 22, 370–384 (2018).
Bingel, U., Schoell, E., Herken, W., Buchel, C. & May, A. Habituation to painful stimulation involves the antinociceptive system. Pain 131, 21–30 (2007).
Schmidt, K., Schunke, O., Forkmann, K. & Bingel, U. Enhanced short-term sensitization of facial compared with limb heat pain. J. Pain 16, 781–790 (2015).
Ellerbrock, I., Wiehler, A., Arndt, M. & May, A. Nocebo context modulates long-term habituation to heat pain and influences functional connectivity of the operculum. Pain 156, 2222–2233 (2015).
McCracken, L. M., Zayfert, C. & Gross, R. T. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain 50, 67–73 (1992).
Walter, B., Hampe, D., Wild, J. & Vaitl, D. Die Erfassung der Angst vor Schmerzen: Eine modifizierte deutsche Version der Pain Anxiety Symptoms Scale (PASS-D). Der Schmerz 16 (2002).
Sullivan, M. J. L., Bishop, S. R. & Pivik, J. The pain catastrophizing scale: development and validation. Psychol. Assess. 7, 524–532 (1995).
Lautenbacher, S. et al. Hypervigilance as predictor of postoperative acute pain: its predictive potency compared with experimental pain sensitivity, cortisol reactivity, and affective state. Clin. J. pain. 25, 92–100 (2009).
Ruscheweyh, R., Marziniak, M., Stumpenhorst, F., Reinholz, J. & Knecht, S. Pain sensitivity can be assessed by self-rating: development and validation of the pain sensitivity questionnaire. Pain 146, 65–74 (2009).
Radloff, L. S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401 (1977).
Hautzinger M. & Bailer, M. Allgemeine Depressionsskala. Weinheim: Beltz (1993).
Laux, L., Hock, M., Bergner-Köther, R., Hodapp, V. & Renner, K. STADI—Das State-Trait-Angst-Depressions-Inventar. Hogrefe (2013).
Lovibond, P. F. & Lovibond, S. H. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behav. Res. Ther. 33, 335–343 (1995).
RStudio-Team. RStudio: integrated development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com42, 14 (2015).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. Stat. Softw. 67, 1–48 (2014).
Boucsein, W. et al. Publication recommendations for electrodermal measurements. Psychophysiology 49, 1017–1034 (2012).
Ishihara, K. & Miyata, Y. [Skin potential activity under two-electrode electrolytes]. Shinrigaku kenkyu 51, 291–294 (1980).
Schmidt, K. https://osf.io/gnk65/?view_only=dcbb22550e684a14bb3a31490ed0c6ae. (2022).
Acknowledgements
This study was funded by the German Research Foundation—project A11, project number 316803389—SFB 1280 (Gefördert durch die Deutsche Forschungsgemeinschaft (DFG)—Projekt A11, Projektnummer 316803389—SFB 1280).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.v.d.S. designed research, analyzed data, and wrote the manuscript. K.S. designed research, analyzed data, and wrote the manuscript. J.S. performed research and wrote the manuscript. M.G. analyzed the data and wrote the manuscript. K.W. analyzed the data and wrote the manuscript. K.F. acquired funding, designed research, analyzed data, and wrote the manuscript. U.B. acquired funding, designed research, analyzed data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Stefano Palminteri and Luke R. Grinham.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Schaaf, M.E., Schmidt, K., Kaur, J. et al. Acquisition learning is stronger for aversive than appetitive events. Commun Biol 5, 302 (2022). https://doi.org/10.1038/s42003-022-03234-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-022-03234-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.