Abstract
Many marine organisms are exposed to decreasing O2 levels due to warming-induced expansion of hypoxic zones and ocean deoxygenation (DeO2). Nevertheless, effects of DeO2 on phytoplankton have been neglected due to technical bottlenecks on examining O2 effects on O2-producing organisms. Here we show that lowered O2 levels increased primary productivity of a coastal phytoplankton assemblage, and enhanced photosynthesis and growth in the coastal diatom Thalassiosira weissflogii. Mechanistically, reduced O2 suppressed mitochondrial respiration and photorespiration of T. weissflogii, but increased the efficiency of their CO2 concentrating mechanisms (CCMs), effective quantum yield and improved light use efficiency, which was apparent under both ambient and elevated CO2 concentrations leading to ocean acidification (OA). While the elevated CO2 treatment partially counteracted the effect of low O2 in terms of CCMs activity, reduced levels of O2 still strongly enhanced phytoplankton primary productivity. This implies that decreased availability of O2 with progressive DeO2 could boost re-oxygenation by diatom-dominated phytoplankton communities, especially in hypoxic areas, with potentially profound consequences for marine ecosystem services in coastal and pelagic oceans.
Similar content being viewed by others
Introduction
Hypoxic waters (defined as having dissolved O2 < 63 μM or 2 mg L−1) occur naturally in both open ocean and nearshore waters, and global warming, as well as anthropogenic eutrophication, have been increasing in their spatial extent and severity1,2,3,4. While hypoxia has often been considered exclusive to deeper waters, near-surface hypoxic waters (< 20 m) are often observed in estuaries5, coastal waters6, and upwelling regions7. Deoxygenation (DeO2) in these areas is predicted to accelerate with progressive ocean global changes, mainly due to ocean-warming8. Decreases in the dissolved O2 content of coastal seawaters are principally due to the heterotrophic degradation of dissolved organic matter associated with coastal eutrophication, resulting in low O2, low pH, and high CO2 conditions9,10,11. While such changes are measured in bulk seawater, their levels are not the same as those in the diffusion boundary layer (DBL) at the photosynthetic cell surface, but nonetheless modeling and direct measurement suggest that changes in the DBL exhibit the same trends, maintaining higher CO2 (lower pH) under elevated CO2 conditions or lower O2 under reduced O2 conditions12,13. Therefore, reduced O2 availability and increased CO2 (lowered pH) in seawater are co-varying drivers in the context of DeO2 and ocean acidification (OA)14. This combination has the potential to disturb the balance between photosynthetic energy supply and respiratory energy consumption in marine ecosystems, and can thus disrupt ecological services3,15.
Photosynthesis of phytoplankton is a major biogeochemical process that oxidizes the oceans, especially by the diatoms that have been estimated to contribute up to 52% of marine O2 production16 and that dominate the phytoplankton communities in hypoxic regions17. Photosynthesis of some diatoms appears to decrease with increased ratios of O2 to CO2 availability18, because carboxylation and oxygenation are catalyzed simultaneously by the central enzyme of photosynthesis ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), and these two reactions compete with each other at the active site of the enzyme to fix CO2 and to consume O2, respectively19. In common with most other phytoplankton, diatoms use energy-costly CO2 concentrating mechanisms (CCMs)20 to increase intracellular CO2 around the active site of Rubisco, minimizing competition from O2 and favoring efficient carboxylation19. It has been shown that increased seawater pCO2 at the levels projected for the end of this century can decrease CCM activity in diatoms and other microalgae21,22 and repress expression of CCM-related genes23,24. The energy savings and resources freed up from downregulation of the CCMs under elevated CO2 conditions could potentially increase primary production under low light levels20,21,25,26. However, under high light levels, excess photochemical energy has been suggested to act with acidic stress to enhance photoinhibition and therefore decrease primary productivity in surface phytoplankton communities26.
It is usually accepted that higher levels of chlorophyll a (Chl a) abundance are positively correlated with high primary productivity27. However, primary productivity per volume of water does not reflect photosynthetic activity or light use efficiency per Chl a, since higher O2 and low pCO2 are often found in waters of high Chl a concentrations28, and are supposed to reduce carboxylation or photosynthetic efficiency as aforementioned. These previous theoretical inferences18 along with our own fieldwork shown here and other observations showing higher levels of phytoplankton photosynthetic efficiency or biomass density in low O2 waters29 led us to hypothesize that a decreased pO2:pCO2 ratio in estuarine and coastal waters could enhance marine productivity and, that this effect of deoxygenation is due to differentially influenced physiological performances of CCMs and photorespiration, which together could act to increase diatom growth rates. OA entails both increased CO2 availability and acidic stress, and so may either decrease or increase photosynthetic efficiency and growth in diatoms, depending on taxonomic differences and environmental conditions18,26,30,31,32. In contrast, the interactions of increased CO2 and reduced O2 on phytoplankton have rarely been considered18. We present here a test of our hypothesis using a series of mesocosm and laboratory experiments that determined the combined effects of elevated CO2 and decreased O2 availability on diatom growth and photosynthesis.
Results
Field investigation
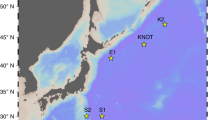
The aim of our field study was to examine whether photosynthetic activity correlates with levels of dissolved O2 (DO), a factor that has seldom been considered in the context of potential effects on oceanic primary productivity. Accordingly, environmental parameters that may influence photosynthetic carbon fixation were investigated at eight different stations in the Pearl River estuary (Fig. 1a, details in Supplementary Table 1). Photosynthetic light use efficiency [PLUE, μmol C (μg Chl a)-1 h-1 (μmol photons m-2 s-1)-1)] was derived from photosynthetic carbon fixation rates measured at low levels (photosynthesis-limiting, <100 and <60 μmol photons m−2 s−1 at 10 and 20 m, respectively) of incident sunlight. PLUE was significantly correlated with DO, CO2 and pH (Fig. 1b–d, Supplementary Table 2, P < 0.0001, r = −0.6120, 0.6589, −0.6463, respectively), but was unrelated to concentrations of dissolved inorganic nitrogen (DIN, NO3− + NO2− + NH4+) and SiO32− (Fig. 1e, f and Supplementary Table 2, P = 0.6671, 0.0707, respectively, Pearson Correlation Analysis). There was an obvious significant increase in PLUE with decreased DO. However, this negative correlation might be attributed to the positive effects of increased CO2 availability and other environmental factors (Fig. 1 and Supplementary Table 1). Therefore, we employed Partial Correlation Analysis to further exclude disturbance from other environmental factors on the correlation between DO and PLUE (see “Data Analysis” for details). These results again indicate a significant correlation of higher PLUE with lower DO (Supplementary Table 2, P = 0.0035, r = −0.4736), suggesting that DO could be one of the key drivers altering in situ photosynthesis and primary production.
Photosynthetic light use efficiency [μmol C (μg Chl a)−1 h−1 (μmol photons m−2 s−1)−1] of phytoplankton assemblages in stations of Pearl River Estuary (a) as a function of dissolved O2 levels (mg L−1) (b), CO2 levels (μM) (c), pHT (d), DIN (NO3− + NO2− + NH4+, μM) (e) and SiO32− (μM) (f). Samples were collected from 10 m (Green) and 20 m (Purple) depths in stations (Blue) of the Pearl River Estuary in the northern South China Sea (June 2015), detailed parameters for the field observations at the stations are shown in the Supplementary Table 1. Significant (P < 0.0001) negative (b, d) and positive (c) correlations and non-significant (e, f, P = 0.6671, 0.0707, Pearson correlation analysis, two-tailed) relationships with O2 (b), CO2 (c), pHT (d), DIN (e) and SiO32− (f) are shown in Supplementary Table 2.
Natural phytoplankton assemblage mesocosm experiments
To test the responses of a natural coastal phytoplankton assemblage to different pO2:pCO2 combinations, we conducted a 30-liter mesocosm experiment under natural levels of sunlight and temperature (Supplementary Fig. 1a) with filtered (180 μm) seawater. While DO, CO2 levels, and pH varied over time, DO and CO2 remained significantly different between the low and high treatments (Supplementary Fig. 1b–d, P < 0.0001). Macronutrients in the mesocosms were consumed rapidly and became depleted within 5 days (Supplementary Fig. 2), with faster removal of the nutrients under low O2 conditions. This was especially obvious for NOx and SiO32- (Supplementary Fig. 2a, c). In contrast, concentrations of chlorophyll a (Chl a) in the mesocosms increased rapidly and peaked within 3 days then declined, with higher concentrations of Chl a under low O2/high CO2 treatments at day 3 (Supplementary Fig. 2d, P = 0.0414, 0.1547 for LOAC and LOHC, respectively).
During the mesocosm experiment, the net and gross photosynthetic rates were higher in the low O2 (LO)-grown than in the ambient O2 (AO)-grown phytoplankton assemblage under both ambient (AC) and high (HC) CO2 levels (relative changes are presented in Fig. 2a–d, absolute values in Supplementary Table 3 and specific p values in Supplementary Table 4), and these enhancements increased with time when NOx stocks diverged between the LO and AO treatments in the mesocosms (Fig. 2a–d and Supplementary Fig. 2a). Under AC, reduced O2 availability significantly enhanced the net photosynthetic rate per volume of seawater (Fig. 2a) at day 3 and day 5 (P = 0.0102, 0.0124), and such significant enhancement was also observed under high CO2 at day 1, 3, 5 (P = 0.0416, 0.0076, 0.0040). Similar trends were also found in Chl a-normalized net photosynthesis (Fig. 2b) under both LOAC and LOHC treatments, though significant enhancement was only observed at day 5 (P = 0.0145, 0.0359) and marginally significant enhancement at day 10 (P = 0.0529 for LOAC). Likewise, gross photosynthetic rates regardless of normalization units and CO2 levels were higher under reduced O2 levels (Fig. 2c, d). Elevated CO2 and the associated pH drop appeared to run counter to the stimulating effects of reduced O2, with lower mean values of photosynthetic rate in the LOHC treatment compared with the LOAC treatment under both normalized units (per water volume or per Chl a), but this was not statistically significant (Fig. 2a–d, P = 0.1270–0.9180, detailed P values in Supplementary Table 4).
(a) Net photosynthesis per volume of seawater (μmol C L−1 h−1), and (b) per Chl a (μmol C (μg Chl a)−1 h−1) measured at day 1, 3, 5, 10, as well as (c) gross photosynthesis per volume of seawater (μmol C L−1 h−1), and (d) per Chl a (μmol C (μg Chl a)−1 h−1) measured at day 3, 5, 10. In a–d, values are presented as % of rates under ambient CO2 and O2 levels, and the absolute values for the rates are shown in Supplementary Table 3. (e) Nonphotochemical quenching (NPQ) measured during the noon period at day 4, 8, 10. Black symbols represent ambient O2 (AO, ~213 μM) and red symbols low O2 (LO, ~57 μM); Circles represents ambient CO2 (AC, ~13 μM); triangles represent high CO2 (HC, ~27 μM). Mesocosms were incubated under incident sunlight and natural levels of temperature (Supplementary Fig. 1), and all data were obtained under growth conditions. Detailed information for the mesocosms experimental features are given in Supplementary Figs. 1 and 2. The values are the means with error bars indicating standard deviations of independent biological replicates (n = 3 mesocosms). Light-colored symbols are individual data corresponding to the treatments. Blue * and red * indicate significant differences (P < 0.05, LSD test) due to low O2 under ambient (LOAC) and elevated CO2 levels (LOHC), respectively, compared to the control treatment (AOAC).
Reduced O2 availability decreased nonphotochemical quenching (NPQ), an indicator of photosynthetic energy loss as heat dissipation and a signal of light stress (Fig. 2e), though only marginally significant changes were observed at day 4 (P = 0.0531 for LOAC and P = 0.086 for LOHC) and day 8 (P = 0.0552, 0.0932). In parallel, reduced O2 level increased photochemical yield (Yield, reflecting all processes downstream of PSII) and effective functional absorption cross-section (σPSII´, an indicator of the efficiency of light capture) during the mesocosm experiment (Fig. 3, detailed P values in Supplementary Table 5). At day 4, reduced O2 increased the Yield significantly (Fig. 3a, P = 0.0002–0.0483) or marginally significantly (P = 0.0664–0.0813) under both CO2 levels, except at 15:00 (P = 0.1377 for LOAC). Meanwhile, reduced O2 significantly (Fig. 3b, P = 0.0004–0.0398) or marginally significantly (P = 0.0557–0.0780) enhanced σPSII´ regardless of CO2 levels except at 08:00 (P = 0.3236 for LOAC and P = 0.3847 for LOHC), 12:00 (P = 0.2962 for LOAC) and 16:00 (P = 0.7898 for LOAC). Similar trends in these measurements were observed both on days 8 and 10 (Fig. 3c–f). These results suggested an enhanced energy transfer in LO-grown phytoplankton.
Effective PSII quantum yield (a, c, e) and the effective functional absorption cross-section of PSII (b, d, f) at days 4, 8, and 10, respectively. Blue dots represent diel changes in photosynthetically active radiation (PAR, μmol photons m−2 s−1) during the experiment. Black symbols represent ambient O2 (AO, ~213 μM) and red symbols low O2 (LO, ~57 μM); circles represent ambient CO2 (AC, ~13 μM); triangles represent high CO2 (HC, ~27 μM). Detailed information for the mesocosms experimental features are given in Supplementary Figs. 1 and 2. The values are the means and the error bars represent standard deviations of independent biological replicates (n = 3 mesocosms). Light-colored symbols are individual data corresponding to the treatments. Blue * and red * indicate significant differences (P < 0.05, LSD test or Games–Howell test) caused by low O2 under ambient (LOAC) and elevated CO2 levels (LOHC), respectively, compared to the control treatment (AOAC).
Based on the CHEMTAX analysis, the phytoplankton community composition changed with time under the different O2 and CO2 combination treatments (Fig. 4). The diverse phytoplankton community was originally dominated by diatoms, cryptophytes, and prasinophytes, but then shifted to have higher proportions of dinoflagellates and the pico-cyanobacterium Synechococcus (Fig. 4) when nutrients were depleted (Supplementary Fig. 2). While diatoms continued as one of the dominant groups throughout the incubation period, the proportion of dinoflagellates obviously increased in the LOAC treatment (Fig. 4c–e, P = 0.0240, 0.0029, 0.0035, correspondingly). A similar trend was found in the LOHC treatment, although a significant increase was only observed at day 5 (Fig. 4d, P = 0.0405).
Proportions of different major phytoplankton groups are indicated in different colors on (a) day 0, (b) day 1, (c) day 3, (d) day 5, (e) day 6, and (f) day 10 under ambient (AO, ~213 μM) and low O2 levels (LO, ~57 μM) with ambient (AC, ~13 μM) and elevated CO2 levels (HC, ~27 μM). D0 represents the initial time of the experiment (22:00, December 27, 2018). Values represent the means of independent biological replicates (n = 3 mesocosms). Detailed information for the mesocosms experimental features are given in Supplementary Figs. 1 and 2. Blue * and red * indicate significant differences in proportions of dinoflagellates (P < 0.05, LSD test) caused by low O2 under ambient (LOAC) and elevated CO2 levels (LOHC), respectively, compared to the control treatment (AOAC).
Diatom culture experiment
Based on the field investigation and mesocosm experiment where diatoms were dominant, a diatom culture experiment was conducted to investigate photosynthetic performance, growth rate, and CCM efficiencies in the globally distributed coastal diatom Thalassiosira weissflogii. The cells were grown under four pO2:pCO2 combinations for over nine generations in laboratory culture. DO, carbonate chemistry and cell numbers were maintained in a stable range (with ~1000–5000 cells mL−1, Supplementary Fig. 3) by diluting the medium every 24 h without using aeration. Levels of DO, pHT, and CO2 in low O2 (LO) and high CO2 (HC) culture conditions differed from those in the ambient O2 (AO) and ambient CO2 (AC) treatments (Supplementary Fig. 3e, f and Supplementary Table 6).
Reduced O2 levels significantly promoted net photosynthesis of the diatom by ~14% under both AC and HC levels (Fig. 5a, P = 0.0024, 0.0005). The absolute rates were higher by ~31% in the AOHC and by ~50% in the LOHC compared with the AOAC treatment (Fig. 5a, P < 0.0001, 0.0001), respectively. Decreased O2 concentration also increased the growth rate by ~14% under AC and only by 9% under HC (Fig. 5b, P < 0.0001, 0.0001). This suggests that there was substantially less enhancement of growth by reduced O2 under the influence of elevated CO2 with lowered pH.
(a) Net photosynthetic rates, (b) specific growth rates (μ), and (c) mitochondrial respiration rates of the cells grown and measured under ambient (AO, ~255 μM) and low O2 levels (LO, ~57 μM) with ambient (AC, ~15 μM) and elevated CO2 levels (HC, ~35 μM). The values are the means and the error bars represent standard deviations of independent biological replicates (n = 3 independent cultures). Light-colored symbols are individual data corresponding to the treatments. Different letters above the bars represent significant differences (P < 0.05, LSD test) among treatments. Detailed information for the experimental features and timing points for the above determinations are shown in Supplementary Fig. 3.
Decreased O2 levels reduced mitochondrial respiration under the AC and HC levels by 41% and 68%, respectively (Fig. 5c, P = 0.0054, P < 0.0001), suggesting that mitochondrial respiration was suppressed by reduced O2 availability to a much greater extent under HC conditions. At the same time, LOAC- and LOHC-grown cells exhibited unchanged high values of photochemical efficiency compared with the cells grown under the AOAC treatment (Supplementary Fig. 4a, P = 0.4717, 0.9663). This indicates that the cells were maintaining a healthy physiological state with high light use efficiency. NPQ decreased significantly in low O2 treatments by 20% (P = 0.0016) and 32% (P < 0.0001) under AC and HC levels, respectively (Supplementary Fig. 4b), suggesting a more efficient energy transfer in LO-grown cells, which is consistent with the results from the mesocosm experiment using natural phytoplankton assemblages (Fig. 2e).
To explore the mechanisms involved, we tested the CCM capacity of the diatom cells acclimated to different combinations of pO2 and pCO2 using direct comparisons under standard conditions (pHT = 8.00, 50–200 μM O2). The LOAC-grown cells had a significantly lower half-saturation constant (K0.5) for CO2-dependent photosynthesis (Supplementary Fig. 4c and Fig. 6a, P < 0.0001), indicating an increased photosynthetic affinity for CO2 and an increase in CCM activity. Conversely, the HC-acclimated cells grown under both AO and LO levels had lower CO2 affinities and CCM activities, as revealed by their increased K0.5 values compared to the AOAC-grown cells (Supplementary Fig. 4c and Fig. 6a, b, P = 0.0038, P < 0.0001). The efficiency of CO2 acquisition, expressed here as the quotient of maximal photosynthetic rate (Vmax) to K0.5, increased significantly with decreased O2 by up to 187%, under the AC level (Fig. 6a, inset, P = 0.0001), but only by about 40% under the HC level (Fig. 6b, inset, P = 0.0481). This implies opposing effects of reduced O2 and elevated CO2 (lowered pH) on CO2 acquisition efficiency.
Net photosynthesis vs CO2 concentration curves was compared under standard conditions (pHT = 8.00, light of 400 μmol photons m−2 s−1, and DO of 50–200 μM) for cells grown under (a) ambient CO2 (AC, circles, ~15 μM) and (b) high CO2 (HC, triangles, ~35 μM) at ambient (AO, black symbols, ~255 μM) and low O2 levels (LO, red symbols, ~57 μM). Insets present CO2 acquisition efficiencies (Vmax/K0.5 for CO2). (c) Photorespiration of cells under the growth pCO2 and pO2 levels. (d) The correlation between photorespiration and CO2 acquisition efficiency (P = 0.0023, r = −0.7886, Pearson correlation analysis, two-tailed). The values represent means (a–c) or all replicate data (d), and error bars indicate the standard deviations of independent biological replicates (n = 3). Light-colored symbols are individual data corresponding to the treatments. Different letters above the bars represent significant differences (P < 0.05, LSD test) among the treatments. The detailed information for the experimental features and timing points for the above determinations are shown in Supplementary Fig. 3.
It appeared that the LO-acclimated cells increased their photorespiration when measured under the standard conditions (nearly ambient O2 level) that at least partly repressed net photosynthetic O2 evolution (Figs. 5a and 6a, b). To check if the divergences between conditions for physiological tests and for experimental cultures may potentially make the observed CCM-related photosynthetic traits under the standard conditions inaccurately reflect those under growth conditions, we examined the activity of periplasmic carbonic anhydrase (eCA) involved in the extracellular conversion of bicarbonate to CO2 using acetazolamide (AZ, as an inhibitor of eCA). The inhibition of photosynthetic O2 evolution by AZ measured under culture conditions was taken as a proxy of eCA-functional capacity and CCM activity (a greater inhibition of eCA relates to higher involvement of biophysical CCMs in photosynthesis). Inhibition was significantly greater in the LO-grown cells compared to AO-grown ones (Table 1, P = 0.0239). This indirectly supports the results showing that lowered O2 concentration enhanced activity of the CCMs and CO2 acquisition efficiency (Supplementary Fig. 4c and Fig. 6a, b). In addition, under the HC conditions AZ had an insignificant effect on the cells grown under both O2 levels (Table 1), as revealed by unchanged net photosynthetic rates under both AO (P = 0.4982) and LO (P = 0.3838) conditions with AZ compared with that without AZ. This reflects that the elevated CO2 alone was sufficient to cause downregulation of eCA and activity of CCMs, regardless of O2 levels, leading to undetectable AZ impacts.
Photorespiration of the diatom declined significantly (42%) in the LO-grown cells under AC levels (P = 0.0058), but decreased to a much lesser extent (20%) in cells grown under LO and HC levels (Fig. 6c, P = 0.0637). Once again, the effects of elevated CO2 were opposite to the positive influence of reduced O2. Photorespiration correlated inversely with CO2 acquisition efficiency (Fig. 6d, P = 0.0023, r = −0.7886), implying a shift from oxygenation to carboxylation catalyzed by ribulose-1,5-bisphosphate due to low O2 enhanced CCMs activity.
On the other hand, reduced O2 availability under AC slightly increased the production rates of particulate organic carbon (POC), particulate organic nitrogen (PON), and biogenic silica (BSi) by 5%, 9%, and 9%, respectively (Supplementary Table 7, P = 0.3247, 0.1102, 0.0057). In comparison, under HC conditions, a reduced O2 level increased POC, PON, and BSi production respectively by 12%, 13%, and 13% (P = 0.0453, 0.3586, 0.0024, respectively). The C:N ratios of the cells were not altered by the LO treatments regardless of the CO2 levels (P = 0.5345, 0.9254).
Discussion
We found that reduced levels of dissolved O2 increased primary productivity of natural phytoplankton assemblages and stimulated growth and enhanced photosynthetic performance with increased activity of CCMs in a cultured diatom (Fig. 7). Mechanistically, low O2-enhancement of CCMs activity along with improved light use efficiency and the reduction in photorespiration allow low O2-grown phytoplankton to perform more efficient photosynthetic carbon fixation (Figs. 2 and 5) and result in faster growth in the diatom (Fig. 5). Reduced photorespiration from favored carboxylation may increase the demand for inorganic carbon, and the reduced mitochondrial respiration may result in decreased intracellular CO2 supply through the respiratory pathway and thus enhance the CCM activity of cells grown under low O2 levels. Although the antagonistic effects of increased CO2 projected for the end of this century on CCMs partly canceled out the positive effects of decreased O2 on the diatom (Fig. 6 and Table 1), reduced levels of O2 still significantly promoted their growth even under the elevated CO2 conditions. Suppression of respiratory carbon loss (Fig. 5) might have also contributed to the enhanced growth rates of the low-O2 grown diatom due to suppressed mitochondrial respiration, the rate of which depends on O2 levels13. Especially under high CO2 conditions, low-O2 grown diatoms possessed higher growth rates with lower mitochondrial respiration, implying that the energy saved from the down-regulated CCMs could have supported the energetic demands for growth so that mitochondrial respiration diminished. These findings supported our hypothesis.
Reduced O2 levels enhance CO2 concentrating mechanisms (CCMs) and photosynthetic performance with increased light energy use efficiency in diatoms and phytoplankton assemblages (Figs. 2–6), promoting photosynthetic carbon fixation and O2 evolution. The enhanced photosynthesis and reduced mitochondrial and photorespiration (Figs. 5 and 6) can accelerate the re-oxygenation due to stimulated photosynthetic O2 evolution by up to 193–250% (based on net photosynthetic values of day 5 in Fig. 2 assuming that the photosynthetic quotient is 1.0). In natural environments, low O2-enhanced production of phytoplankton biomass makes them a more effective O2 source that may help to counteract the negative effects of hypoxia on heterotrophs. Black, red, and blue arrows indicate directions, increase and decrease, respectively.
Whether the positive effects of reduced O2 on phytoplankton assemblages observed in this work are true for dynamic in situ environments remains to be explored, in view of possible synergistic or antagonistic effects of multiple drivers. The sensitivity of phytoplankton to O2 can be closely linked to their physiological conditions, types and/or efficiencies of CCMs and Rubisco19,33,34. Thus additional environmental stresses and diverse phytoplankton assemblage structures may complicate overall ecosystem responses35. For instance, changes in nutrient availabilities and phytoplankton communities in our mesocosms under fluctuating levels of PAR and temperature appeared to have affected the interactions of CO2 and O2 (Figs. 2–4). In addition, other components of the plankton communities, such as grazers, might have complicated the interactions within the mesocosm system. These factors may be at least partially responsible for observed differences in the magnitude of low-O2 enhancement effects and high CO2 dampening impacts on photosynthetic carbon fixation.
Most dinoflagellates are characterized by only moderately efficient CCMs and high O2 affinity-form II Rubisco, and therefore may benefit more from reduced O219. This may account for the increased proportion of dinoflagellates in our low O2 mesocosms after nutrients, especially after SiO32− became exhausted. On the other hand, their complex nutritional modes, such as heterotrophic nutrition and phagotrophy, may give dinoflagellates more strategies to withstand low O2 environments. As recently reported, Noctiluca scintillans, which relies on ingested endosymbionts, bloomed during a hypoxic event in the Arabian Sea36. The aforementioned positive effects of lowered O2 and multiple nutritional modes might have increased the abundance of dinoflagellates encountering hypoxic waters. This implies that hypoxic waters or ocean deoxygenation could enhance the development of harmful dinoflagellate blooms.
As global warming and eutrophication have perturbed the O2 budget of the ocean, degradation of habitat fitness for aerobic marine organisms has occurred both regionally and globally3,4,8. Importantly, recently reported time-series data suggest the occurrence of upwelling-induced continuous hypoxia events (~1–2 weeks) in shallower layers37. In our study, however, natural phytoplankton assemblages and the diatom T. weissflogii benefited from reduced O2 concentrations that were low enough to be detrimental for most marine animals15,38. Accordingly, even under elevated CO2 conditions, low O2-enhanced photosynthesis can accelerate “re-oxygenation” in illuminated waters by ~193–250% (based on the net photosynthetic values of day 5 in Fig. 2, and an assumption that the photosynthetic quotient is 1.0), and thus may progressively alleviate the impacts of diminished oxygen on animals (Fig. 7). Considering that open ocean diatoms are more sensitive to rising CO2 than coastal ones39, the combined impacts of reduced O2 and increased CO2 levels on coastal and pelagic phytoplankton taxa are expected to differ in extent. Thus, the present result, showing that lowered availability of O2 enhanced primary production of phytoplankton, indicates a possible negative feedback effect on ocean deoxygenation.
Marine primary producers are exposed to multiple stressors along with progressive ocean acidification (OA) and warming40, being affected by enhanced nutrient limitation in pelagic waters and by deteriorating eutrophication in coastal areas, along with ocean-warming-induced decrease in oxygen solubility. Ocean deoxygenation has been predicted to cause a further 1–7% decline in the global ocean O2 inventory over this century, due to global warming8. Moreover, increasing discharges of nitrogen and phosphorus to coastal waters41 and strengthening upwelling-favorable winds42 may make invasions of hypoxic waters into the euphotic zone happen more frequently. This has been suggested to intensify the combination of DeO2 and OA effects, especially in coastal regions4,43,44. With progressive ocean climate changes, DeO2 is believed to disrupt the balance between O2 availability and metabolic O2 demand of some marine biota and impact heterotrophic processes3. Thus, climate change (such as warming) may increase the energy demand of aerobic organisms while DeO2 reduces the O2 supply. However, both DeO2 and OA occur in concert with other environmental drivers and biological factors. Therefore, it is important to note that the results from our laboratory and mesocosm experiments can only provide a mechanistic understanding of the positive effects of lowered O2 under influence of elevated CO2 (Fig. 7). Other key biological responses under multiple drivers along with long-term selection and evolution of dominant phytoplankton to life under low O2/high CO2 conditions are unknown, but should be a priority for further research. Future studies on the ocean deoxygenation effects are also encouraged to include more drivers, to better reflect the real complexities of future ocean environments.
Methods
Field studies
Photosynthetic carbon fixation was investigated at eight different stations in the Pearl River estuary of the South China Sea (Fig. 1a and Supplementary Table 1), where the phytoplankton assemblages were dominated by diatoms45 during the time of our investigation (June 2015). Samples were collected from 10 to 20 m depths and transferred immediately into 50 mL quartz tubes and sealed to prevent gas exchange. The samples were inoculated with 100 μL of 5 μCi (0.185 MBq) NaH14CO3 solution for 2.15 h. All the incubations were carried out under incident solar radiation, attenuated with neutral density filters to simulate light intensities at the sampling depths, and the temperature was controlled with flow-through surface seawater.
After incubation, the cells were filtered onto glass-fiber filters (25 mm, Whatman GF/F, USA) and stored at −20 ° C until measurement, during which the filters were exposed to HCl fumes overnight and dried (20 °C, 6 h) to remove unincorporated NaH14CO3 as CO2. The incorporated radioactivity was measured by liquid scintillation counting (LS 6500, Beckman Coulter, USA), and photosynthetic carbon fixation rates were estimated as previously reported46. Since the measurements were carried out under varying and low light levels similar to in situ levels at depths of 10 and 20 m, we normalized the photosynthetic rates to light intensity (μmol C (μg Chl a)−1 h−1 (μmol photons m−2 s−1)−1) to obtain the light use efficiency of photosynthesis (PLUE). This was done to allow for a meaningful comparison among different stations according to the linear relationship of photosynthetic carbon fixation under low solar irradiance levels46, which lies within the range of sunlight levels used in the present fieldwork (<100 μmol photons m−2 s−1).
Field DO, chlorophyll a (Chl a) concentration and nutrients were measured as described previously5,47. Briefly, field DO was manually measured on board using the Winkler titration method48. The Chl a content was measured with a Turner Designs Model 10 Fluorometer. The nitrogen (NOX, NO3− + NO2−), NH4+, and SiO32− concentrations were measured with a nutrient-autoanalyzer (Quickchem 8500, Lachat Instruments, USA) following the description of Kirkwood et al.49. This equipment has detection limits of 0.014 and 0.075 μM for NOX and SiO32−, respectively.
Dissolved inorganic carbon (DIC) concentrations at investigated stations were estimated based on measured salinity and the relationship between salinity and DIC concentrations in the published literature50 in the same area of the Pearl River estuary during the same season. CO2 concentration and pHT were calculated using CO2SYS software51, using the equilibrium constants K1 and K2 for carbonic acid dissociation52.
Mesocosm studies
Surface seawater (0–1 m) with natural plankton assemblages was sampled from a harbor near the Dongshan Swire Marine Station of Xiamen University (23.65o N, 117.49o E) with an acid-cleaned plastic bucket, filtered (180 μm) to remove large grazers, and transported to the station within 1 h. The incubation system used 30-liter cylindrical polymethyl methacrylate tanks (n = 3), which allowed 91% PAR transmission and were water-jacketed for temperature control with a re-circulating cooler (running water). We set two O2 and two CO2 levels with three pO2:pCO2 combinations: (1) ambient O2 (AO, ~213 μM) & ambient CO2 (AC, ~13 μM), AOAC; (2) low O2 (LO, ~57 μM) & ambient CO2, LOAC; (3) low O2 & high CO2 (HC, ~27 μM), LOHC. The presented O2 and CO2 concentrations are average values across the entire experiment. N2, CO2, and air were mixed proportionally to create different and stable pO2:pCO2 combinations in the gas stream. The incubation tanks were continuously aerated (0.5 L min−1) under incident solar radiation. The O2 concentration was measured (20:00) with a precise single-channel fiber optic oxygen sensor (Microx 4, PreSence, Germany) every day. CO2 concentrations of seawater were calculated from daily measured pHNBS (20:00) and TA measured every other day using CO2SYS software. The pH was determined according to Dickson (2010)53 with a high-quality pH meter (Orion StarA211, Thermo, USA) which was calibrated with standard National Bureau of Standards (NBS) buffer solutions (Hanna). The pHNBS values were converted to pHTotal (pHT) using the CO2SYS software as described above.
For nutrient measurements, water samples were stored in 80-mL polycarbonate bottles, instantly frozen, and stored at −20 °C until analysis. Samples for silicate determination were fixed with 1‰ chloroform and preserved at 4 °C. Nutrients were measured with an AA3 Auto-Analyzer (Bran-Luebbe, GmbH, Germany) with detection limits of 0.08, 0.08, and 0.16 μM for NOX, PO43−, and SiO32−, respectively.
Samples for analysis of Chl a and other pigments were filtered onto glass-fiber filters (25 mm, Whatman GF/F, USA) which were immediately preserved in liquid nitrogen until analysis. Measurement was conducted with a high-performance liquid chromatography system (UltiMate 3000, ThermoFisher Scientific, USA) after filters were submerged in N, N-dimethylformamide and then mixed 1:1 (V:V) with 1-M ammonium acetate54. Chlorophyll a and other pigments were identified by their retention times and quantified using peak areas and standard curves. Quantification was performed with standards purchased from DHI Water & Environment, Hørsholm, Denmark. Chemotaxonomic analysis was carried out using CHEMTAX software55,56.
To measure gross and net primary productivity, respectively, seawater samples were inoculated with 200 μL of 10 μCi (0.37 MBq) NaH14CO3 solution (ICN Radiochemicals, USA) for 2 h (gross) and with 100 μL of 5 μCi (0.185 MBq) NaH14CO3 solution for 24 h (net). All the incubations were carried out under incident solar radiation in a flow-through water bath to obtain a uniform temperature. Photosynthetic carbon fixation rates in the mesocosm experiment were estimated as described above.
Photosynthetic fluorescence parameters were measured with a fluorescence induction and relaxation system (In-Situ FIRe, Satlantic, NS Canada). NPQ was estimated by the equation of Genty et al.57:
where Fmd is the maximal fluorescence measured before sunrise and Fm’ is the effective yield at 11:00 a.m. under incident sunlight.
Diatom culture studies
The diatom Thalassiosira weissflogii (CCMP 1336) was incubated in artificial seawater prepared according to the Aquil* medium recipe58, and was cultured semi-continuously in polycarbonate bottles. Cultures were incubated at 20 °C in a plant growth chamber (HZ100LG, Ruihua, Wuhan, China) and illuminated with cool white fluorescent light at 200 μmol photons m−2 s−1 (measured by a US-SQS/WB spherical micro quantum sensor; Walz, Germany) with a 12:12 h light:dark cycle. The maximum cell concentration was maintained below 5000 cells mL-1 by diluting the cultures every 24 h with newly prepared medium, equilibrated with the target O2 and CO2 levels, in order to maintain a stable range of dissolved O2 (DO) and carbonate chemistry in the culture without aeration (Supplementary Fig. 3). To avoid the cells settling, the bottles were shaken gently every 2 h during the daytime (0800–2000).
The diatom cells were acclimated to four treatments with two levels of CO2 (ambient and high CO2) and two levels of O2 (ambient and low O2), respectively. In order to create the ambient O2 & ambient CO2 seawater (AOAC, ~254 μM O2, ~15 μM CO2) or ambient O2 & high CO2 seawater (AOHC, ~256 μM O2, ~33 μM CO2), we aerated the medium with ambient air or CO2-enriched air using a CO2 enricher (CE-100, Ruihua, Wuhan, China). In order to maintain low O2 conditions and to sustain constant carbonate chemistry, pure nitrogen was introduced into the headspace of bottles containing seawater with different CO2 concentrations, so that the O2 in the water was displaced, and reduced O2 & ambient CO2 (LOAC, ~58 μM O2, ~14 μM CO2) or reduced O2 & high CO2 (LOHC, ~56 μM O2, ~36 μM CO2) conditions were achieved (Supplementary Fig. 3e, f and Supplementary Table 6).
The dissolved O2 and pH of seawater were measured before and after diluting the culture medium (Supplementary Fig. 3 and Supplementary Table 6). The dissolved O2 was measured with a Clark-type oxygen electrode (Hansatech, UK). Parameters of the seawater carbonate system (Supplemental Table 6) were calculated from pH and TA with CO2SYS software, and the pHNBS values were converted to pHTotal (pHT) using the CO2SYS software as described above. Photosynthesis vs CO2 curves (n = 3) and other parameters (n = 3) were obtained from two separate experiments under the same experimental conditions after the cells had acclimated for at least nine generations (see Supplemental Fig. 3 for detail).
Cell concentrations were measured with a Counter Particle Count and Size Analyzer (Z2, Beckman Coulter, USA) before and after the dilutions every 24 h. The cells had acclimated for at least nine generations before the growth rate was measured. The specific growth rate (μ, d−1) was calculated as
where N1 and N0 represent cell concentrations at t1 (before the dilution) and t0 (initial or just after the dilution), respectively.
A Clark-type oxygen electrode was used to measure mitochondrial respiration (after acclimation for ~13 generations) under the conditions of pH, O2 levels, and temperature used for growth, and the oxygen consumption rates were monitored in the dark (~10 min). About 6–8 × 105 cells were harvested by gentle vacuum filtration (<0.01 MPa) onto polycarbonate membrane filters (1.2 μm, Millipore, Germany). These cells were then re-suspended in seawater (2 mL) buffered with 20 mM Tris (without introducing additional DIC into media, pHT = 8.00 for AC of 14 μM and pHT = 7.70 for HC of 34 μM) to maintain stable pH in the media. Tris-buffered seawaters were flushed with pure nitrogen and ambient air to achieve the culture O2 levels.
During the measurements of photosynthetic O2 evolution and photorespiration, 5–6 × 105 cells were harvested after acclimation for ~18 generations and re-suspended as above. Photosynthetic O2 evolution was tested under growth O2 levels (~255 μM for AO and ~57 μM for LO), and photorespiration (Supplementary Fig. 5) was estimated as the difference in photosynthetic O2 evolution of the cells under reduced (~25 μM) and culture (~255 μM for AO and ~57 μM for LO) O2 conditions, an approach which has been used widely26,59. However, this method might have overestimated the absolute value of photorespiration to some extent because of the ignored mitochondrial respiration rates at different O2 levels. Therefore, we re-estimated the photorespiration (Fig. 6c) using the differences of dark-respiration rates between the samples measured under ~25 μM O2 and growth O2 conditions (~255 μM O2 for AO and ~57 μM O2 for LO), assuming that the mitochondrial respiration rates for the cells grown under the treatments were the same under light and darkness. To obtain the reduced or ambient levels of O2, pure nitrogen gas or ambient air were bubbled into Tris-buffered seawater (20 mM, pHT = 8.00 for AC of about 14 μM and pHT = 7.70 for HC of about 34 μM). Light intensity and temperature were the same as in the growth experiment.
Inhibition of photosynthetic O2 evolution by acetazolamide (AZ)60, an inhibitor of periplasmic carbonic anhydrase (eCA), was determined with a Clark-type oxygen electrode under culture conditions. We added the AZ dissolved in 0.05 mM NaOH at a final concentration of 100 μM; an equal amount of 0.05 mM NaOH was added as a control treatment. The cells used for this test had been acclimated to the growth O2 and CO2 levels for about ten generations, and ~5 × 105 cells were harvested and re-suspended in 2 mL seawater buffered with 20 mM Tris to maintain the CO2 partial pressures as mentioned above. O2 levels were achieved and controlled as above.
The photosynthesis vs CO2 curves was determined with a Clark-type oxygen electrode under standard conditions commonly used for CCM studies19. Approximately 4–10 × 105 cells were harvested as above after acclimation for approximately nine generations and were re-suspended in DIC-free seawater (2 mL) medium buffered with 20 mM Tris (pHT = 8.00). The concentrations of DIC in the seawater were then adjusted by adding sodium bicarbonate solution, and the final DIC concentration reached to 8 mM. DIC (μM) values were converted to CO2 (μM) with CO2SYS software. All the cells from different treatments were measured under the same standard conditions (pHT = 8.00, light intensity = 400 μmol photons m−2 s−1, O2 was in the range of 50–200 μM, and the temperature was controlled at 20 ± 0.1 °C). CO2 acquisition efficiency was calculated as
where Vmax and K0.5 were calculated by fitting the photosynthetic O2 evolution rates at various CO2 concentrations with the Michaelis–Menten formula.
Measurements of chlorophyll fluorescence parameters were carried out with a pulse amplitude modulated (PAM) fluorometer (XE-PAM, Walz, Effelrich, Germany) after the cells had acclimated for ~12 generations. Effective photosystem II (PSII) quantum yield of photosystem (Yield) was measured with an actinic light level of 226 μmol photons m−2 s−1 (similar to that of the culture level). Nonphotochemical quenching (NPQ) was also measured at this actinic light intensity.
Approximately 5–8 × 105 cells were harvested (~18 generations) for measuring elemental composition. Particulate organic carbon (POC) and particulate organic nitrogen (PON) were determined by filtering cells on the pre-combusted (450 °C for 6 h) GF/F filters (25 mm, Whatman), storing at −80 °C before measuring. Filters were treated with HCl fumes to remove inorganic carbon and dried before analysis on a CHNS elemental analysizer (vario EL cube, Elementar, Germany). Biogenic silica (BSi) was determined by the spectrophotometric method61, and the cells were harvested onto Polycarbonate filters (1.2 μm, Millipore, Germany). Production of POC, PON, and BSi was calculated by multiplying the cellular content by specific growth rate.
Statistics and reproducibility
The data are expressed in raw form, or presented as means ± standard deviation (SD) with n = 3 (triplicate cultures or mesocosms). We used one-way ANOVA to assess significant differences among the treatments. Prior to analyses, data were checked for homoscedasticity. If required, data were Ln transformed, and then LSD test was used for post hoc investigation. If the data, even after transformation, did not meet the assumption for equal variance, Games–Howell tests were chosen for post hoc investigation. Linear fitting analysis was conducted with Pearson correlation analysis (two-tailed). Partial Correlation Analysis was employed to explore the net correlation between DO and photosynthetic light use efficiency in the Pearl River estuary investigation. Parameters including pHT, cultured temperature, DIN, SiO32−, DIC, and CO2 were under control. A 95% confidence level was used in all analyses.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The source data that underlying the main charts are provided as Supplementary Data 1.
References
Hutchins, D. A. & Fu, F. X. Microorganisms and ocean global change. Nat. Microbiol. 2, 17058 (2017).
Boyd, P. W. et al. Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change—a review. Glob. Change Biol. 24, 2239–2261 (2018).
Breitburg, D. et al. Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240 (2018).
Fennel, K. & Testa, J. M. Biogeochemical controls on coastal hypoxia. Annu. Rev. Mar. Sci. 11, 105–130 (2019).
Li, G. et al. Subsurface low dissolved oxygen occurred at fresh- and saline-water intersection of the Pearl River estuary during the summer period. Mar. Pollut. Bull. 126, 585–591 (2018).
Chen, C.-C., Gong, G.-C. & Shiah, F.-K. Hypoxia in the East China Sea: one of the largest coastal low-oxygen areas in the world. Mar. Environ. Res. 64, 399–408 (2007).
Grantham, B. A. et al. Upwelling-driven nearshore hypoxia signals ecosystem and oceanographic changes in the northeast Pacific. Nature 429, 749–754 (2004).
Keeling, R. F., Körtzinger, A. & Gruber, N. Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci 2, 199–229 (2010).
Cai, W.-J. et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 4, 766–770 (2011).
Gray, J. S., Wu, R. S.-s & Or, Y. Y. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 238, 249–279 (2002).
Wang, B. et al. Diatom bloom-derived bottom water hypoxia off the Changjiang estuary, with and without typhoon influence. Limnol. Oceanogr. 62, 1552–1569 (2017).
Flynn, K. J. et al. Changes in pH at the exterior surface of plankton with ocean acidification. Nat. Clim. Change. 2, 510–513 (2012).
Kim, M. et al. Low oxygen affects photophysiology and the level of expression of two-carbon metabolism genes in the seagrass Zostera muelleri. Photosynth. Res. 136, 147–160 (2018).
Gattuso, J.-P. et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349, aac4722 (2015).
Brewer, P. G. & Peltzer, E. T. Limits to marine life. Science 324, 347–348 (2009).
Rousseaux, C. S. & Gregg, W. W. Interannual variation in phytoplankton primary production at a global scale. Remote Sens. 6, 1–19 (2014).
Estrada, M. & Blasco, D. in International symposium on the upwelling areas off Western Africa (Cape Blanco and Benguela) Vol. 1, (eds Bas, C., Margalef, R. & Rubies, P.) 379–402 (Instituto de Investigaciones Pesqueras, 1985).
Gao, K. & Campbell, D. A. Photophysiological responses of marine diatoms to elevated CO2 and decreased pH: a review. Funct. Plant Biol. 41, 449–459 (2014).
Reinfelder, J. R. Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu. Rev. Mar. Sci. 3, 291–315 (2011).
Hopkinson, B. M., Dupont, C. L., Allen, A. E. & Morel, F. M. M. Efficiency of the CO2-concentrating mechanism of diatoms. Proc. Natl Acad. Sci. USA 108, 3830–3837 (2011).
Wu, Y., Gao, K. & Riebesell, U. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 7, s915–s2923 (2010).
Raven, J. A., Giordano, M., Beardall, J. & Maberly, S. C. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth. Res. 109, 281–296 (2011).
Hennon, G. M. M. et al. Diatom acclimation to elevated CO2 via cAMP signalling and coordinated gene expression. Nat. Clim. Chang. 5, 761–765 (2015).
Shi, D. et al. Interactive effects of light, nitrogen source, and carbon dioxide on energy metabolism in the diatom Thalassiosira pseudonana. Limnol. Oceanogr. 60, 1805–1822 (2015).
Hein, M. & Sand-Jensen, K. CO2 increases oceanic primary production. Nature 388, 526–527 (1997).
Gao, K. et al. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat. Clim. Chang. 2, 519–523 (2012).
Tan, S.-C., Shi, G.-Y., Shi, J.-H., Gao, H.-W. & Yao, X. Correlation of Asian dust with chlorophyll and primary productivity in the coastal seas of China during the period from 1998 to 2008. J. Geophys. Res. Biogeosci. 116, G2 (2011).
Liu, N. et al. Carbon assimilation and losses during an ocean acidification mesocosm experiment, with special reference to algal blooms. Mar. Environ. Res. 129, 229–235 (2017).
Chavez, F. P. & Messié, M. A comparison of eastern boundary upwelling ecosystems. Prog. Oceanogr. 83, 80–96 (2009).
Riebesell, U., Wolf-Gladrow, D. A. & Smetacek, V. Carbon dioxide limitation of marine phytoplankton growth rates. Nature 361, 249–251 (1993).
Hoppe, C. J. M., Holtz, L.-M., Trimborn, S. & Rost, B. Ocean acidification decreases the light-use efficiency in an Antarctic diatom under dynamic but not constant light. New. Phytol. 207, 159–171 (2015).
Passow, U. & Laws, E. A. Ocean acidification as one of multiple stressors: growth response of Thalassiosira weissflogii (diatom) under temperature and light stress. Mar. Ecol. Prog. Ser. 541, 75–90 (2015).
Badger, M. R. et al. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. 76, 1052–1071 (1998).
Ku, S.-B. & Edwards, G. E. Oxygen inhibition of photosynthesis: I. temperature dependence and relation to O2/CO2 solubility ratio. Plant. Physiol 59, 986–990 (1977).
Brennan, G. & Collins, S. Growth responses of a green alga to multiple environmental drivers. Nat. Clim. Chang. 5, 892–897 (2015).
do Rosário Gomes, H. et al. Massive outbreaks of Noctiluca scintillans blooms in the Arabian Sea due to spread of hypoxia. Nat. Commun. 5, 4862 (2014).
Gireeshkumar, T. R. et al. Influence of upwelling induced near shore hypoxia on the Alappuzha mud banks, south west coast of India. Cont. Shelf. Res. 139, 1–8 (2017).
Steckbauer, A., Klein, S. G. & Duarte, C. M. Additive impacts of deoxygenation and acidification threaten marine biota. Glob. Change Biol. 26, 5602–5612 (2020).
Li, F., Wu, Y., Hutchins, D. A., Fu, F. & Gao, K. Physiological responses of coastal and oceanic diatoms of diurnal fluctuations in seawater carbonate chemistry under two CO2 concentrations. Biogeosciences 13, 6247–6259 (2016).
Gao, K. et al. Effects of ocean acidification on marine photosynthetic organisms under concurrent influences of warming, UV radiation, and deoxygenation. Front. Mar. Sci 6, 322 (2019).
Reed, D. C. & Harrison, J. A. Linking nutrient loading and oxygen in the coastal ocean: a new global scale model. Glob. Biogeochem. Cycle 30, 447–459 (2016).
Bakun, A., Field, D. B., Redondo-Rodriguez, A. & Weeks, S. J. Greenhouse gas, upwelling-favorable winds, and the future coastal ocean upwelling ecosystems. Glob. Change Biol. 16, 1213–1228 (2010).
Melzner, F. et al. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888 (2013).
Baumann, H., Wallace, R. B., Tagliaferri, T. & Gobler, C. J. Large natural pH, CO2 and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal, and interannual time scales. Estuaries Coast 38, 220–231 (2015).
Xiao, W. et al. Realized niches explain spatial gradients in seasonal abundance of phytoplankton groups in the South China Sea. Prog. Oceanogr. 162, 223–239 (2018).
Gao, K. et al. Solar UV radiation drives CO2 fixation in marine phytoplankton: a double-edged sword. Plant. Physiol. 144, 54–59 (2007).
Li, J. et al. Spatial and seasonal distributions of bacterioplankton in the Pearl River Estuary: the combined effects of riverine inputs, temperature, and phytoplankton. Mar. Pollut. Bull. 125, 199–207 (2017).
Carpenter, J. H. The Chesapeake Bay Institute technique for the Winkler dissolved oxygen method. Limnol. Oceanogr. 10, 141–143 (1965).
Kirkwood, D. S., Aminot, A. & Carlberg, S. R. The 1994 QUASIMEME laboratory performance study: Nutrients in seawater and standard solutions. Mar. Pollut. Bull. 32, 640–645 (1996).
Li, X. et al. Production and transformation of dissolved and particulate organic matter as indicated by amino acids in the Pearl River Estuary. China. J. Geophys. Res-Biogeo. 123, 3523–3537 (2018).
Lewis, E. & Wallace, D. W. R. Program Developed for CO2 System Calculations, ORNL/CDIAC-105. (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge, TN, US Department of Energy, 1998).
Millero, F. J., Graham, T. B., Huang, F., Bustos-Serrano, H. & Pierrot, D. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar. Chem. 100, 80–94 (2006).
Dickson, A. G. in Guide to best practices for ocean acidification research and data reporting (eds Riebesell, U., Fabry, V. J., Hansson, L. & Gattuso, J.-P.) 17–40 (Publications Office of the European Union, 2010).
Zapata, M., Rodríguez, F. & Garrido, J. L. Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 195, 29–45 (2000).
Mackey, M. D., Mackey, D. J., Higgins, H. W. & Wright, S. W. CHEMTAX- a program for estimating class abundances from chemical markers: application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 144, 265–283 (1996).
Liu, X. et al. Responses of phytoplankton communities to environmental variability in the East China Sea. Ecosystems 19, 832–849 (2016).
Genty, B., Briantais, J.-M. & Baker, N. R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990, 87–92 (1989).
Sunda, W. G., Price, N. M. & Morel, F. F. M. in Algal Culturing Techniques (ed. Andersen, R. A) 35–63 (Elsevier Academic Press, 2005).
Björk, M., Haglund, K., Ramazanov, Z. & Pedersén, M. Inducible mechanisms for HCO3- utilization and repression of photorespiration in protoplasts and thalli of three species of Ulva (Chlorophyta). J. Phycol. 29, 166–173 (1993).
Hopkinson, B. M., Meile, C. & Shen, C. Quantification of extracellular carbonic anhydrase activity in two marine diatoms and investigation of its role. Plant. Physiol. 162, 1142–1152 (2013).
Brzezinski, M. A. & Nelson, D. M. The annual silica cycle in the Sargasso Sea near Bermuda. Deep-Sea Res. 42, 1215–1237 (1995).
Acknowledgements
This study was supported by the national key R&D program (2016YFA0601400), National Natural Science Foundation (41720104005, 41890803, 41721005). K.G and D.H. are grateful to SCOR’s support for their participance in SCOR WG149 meetings. The authors are grateful to the laboratory engineers, Xianglan Zeng and Wenyan Zhao, and to Shengyao Sun and Qisi Cai of Dongshan Swire Station of Xiamen University.
Author information
Authors and Affiliations
Contributions
K.G. and J.-Z.S. contributed to design, plan the experiments, and write the paper. J.-Z.S., R.H., and D.Z. performed the laboratory experiments. T.W., G.L., and X.Y. contributed to the field experiments. J.-Z.S., T.W., X.W., Z.D., and X.L. contributed to the mesocosm experiment. J.B., D.H., and G.G. contributed to the analysis of the data and the writing of the paper. All of the authors contributed data analysis, revisions, and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks John Reinfelder and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editors: Linn Hoffmann and Caitlin Karniski.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, JZ., Wang, T., Huang, R. et al. Enhancement of diatom growth and phytoplankton productivity with reduced O2 availability is moderated by rising CO2. Commun Biol 5, 54 (2022). https://doi.org/10.1038/s42003-022-03006-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-022-03006-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.