Abstract
Heritable microorganisms play critical roles in life cycles of many macro-organisms but their prevalence and functional roles are unknown for most plants. Bioactive ergot alkaloids produced by heritable Periglandula fungi occur in some morning glories (Convolvulaceae), similar to ergot alkaloids in grasses infected with related fungi. Ergot alkaloids have been of longstanding interest given their toxic effects, psychoactive properties, and medical applications. Here we show that ergot alkaloids are concentrated in four morning glory clades exhibiting differences in alkaloid profiles and are more prevalent in species with larger seeds than those with smaller seeds. Further, we found a phylogenetically-independent, positive correlation between seed mass and alkaloid concentrations in symbiotic species. Our findings suggest that heritable symbiosis has diversified among particular clades by vertical transmission through seeds combined with host speciation, and that ergot alkaloids are particularly beneficial to species with larger seeds. Our results are consistent with the defensive symbiosis hypothesis where bioactive ergot alkaloids from Periglandula symbionts protect seeds and seedlings from natural enemies, and provide a framework for exploring microbial chemistry in other plant-microbe interactions.
Similar content being viewed by others
Introduction
Microbial biochemistry plays a protective role in many macro-organisms where symbiotic hosts acquire higher fitness than non-hosts in the presence of natural enemies. This stands in contrast to nutritional symbioses where the microbial symbionts provide limiting resources to the host as in symbiotic N-fixation in legumes and provisioning of essential amino acids in aphids1. In many protective symbioses, the microbial symbionts are transmitted to offspring through the maternal lineage. For example, beewolf wasps (Hymenoptera) harbor antibiotic-producing symbiotic bacteria that protect larvae from fungal infections2, and intracellular bacteria producing kahalalide compounds protect their algal hosts against predation3. In many grasses (Poaceae), heritable fungal symbionts produce bioactive ergot alkaloids with anti-herbivory properties4. These defensive symbioses represent rich sources of natural products, and tractable systems for investigating the dynamics of interspecific interactions. More generally, understanding the origin, distribution and function of heritable symbioses provide insights into the evolution of major groups of eukaryotes.
Some morning glory species (family Convolvulaceae) contain high concentrations of ergot alkaloids (EAs) similar to those found in grasses infected with fungi in the family Clavicipitaceae4,5. EAs, named for ergot fungi (Claviceps spp.), are characterized by a common tetracyclic ergoline structure with a variety of complex side chains, and were first isolated from morning glory seeds in 19606 based on their use as entheogens by indigenous peoples in Mexico7,8. EAs have been of longstanding interest given their toxic effects on humans and animals, medical applications and psychoactive properties9. For example, the EA lysergic acid provides the precursor for lysergic acid diethylamide (LSD). Much previous research has focused on increased herbivore and stress resistance of symbiotic grasses containing these fungal alkaloids10.
Morning glories are commonly considered as species of Ipomoea but this large genus is not monophyletic11, and a more accurate concept of morning glories is members of the tribe Ipomoeeae (morning glories with spiny pollen)12. Species are distributed across the world’s tropical and subtropical regions, are commonly found in disturbed habitats and thrive in open ecotonal areas such as forest and river edges13. Morning glories are important for humans as food crops (I. batatas, sweetpotato; I. aquatica, water spinach), agricultural weeds (I. purpurea, I. triloba) and ornamental flowers (I. alba, I. tricolor), and are an emerging model system for evolutionary studies14.
While it was known that Claviceps and related species produce EAs in their grass hosts, morning glories were initially thought to produce EAs endogenously until researchers15 treated I. asarifolia with fungicide and observed that EAs and characteristic epiphytic fungal colonies disappeared, suggesting that EAs in morning glories are also produced by symbiotic fungi. The EA biosynthetic gene, dmaW, and nuclear rDNA sequences typical of Clavicipitaceae were identified in the fungus16, which infected plants systemically and was vertically transmitted through seeds17,18. Epiphytic fungal colonies can be observed in many symbiotic morning glory species on the adaxial surface of young leaves and closely associated with oil glands, providing a potential pathway for fungal nutrition and horizontal transmission, which has not been experimentally observed. This new fungal genus associated with Convolvulaceae was named Periglandula and genetic analysis grouped the fungi within the Clavicipitaceae19.
Prior to the discovery of Periglandula symbiosis, 42 species of morning glories out of 98 tested5 were reported to contain EAs. All reports were from the tribe Ipomoeeae, which includes the genera Argyreia, Stictocardia, Turbina, and the paraphyletic genus Ipomoea20,21. Two Periglandula species have been described to date based on their host associations and exhibit considerable genetic differentiation between them19. Efforts to cultivate Periglandula have been thus far unsuccessful, suggesting that some host products are necessary for fungal growth. Recent studies22,23,24 reported the co-occurrence of Periglandula, ergot alkaloid synthesis genes, and EAs in seeds of 10 additional morning glory species beyond the initial two described. Notably, Periglandula symbiosis with Convolvulaceae is macroscopically asymptomatic with hereditary (vertical) transmission through seeds and EA production at concentrations up to 1000X higher than in grasses infected with symbiotic Epichloë species. Moreover, morning glories are the only dicotyledonous plants known to contain EAs from fungal symbionts that have no known sexual reproduction, spore production or pathogenic relatives, unlike related Clavicipitaceae of grasses.
Given the vertical transmission of EAs and Periglandula through host seeds, we hypothesize that both symbiosis and EA chemistry will vary among host lineages through evolutionary diversification and speciation, and reflect the ecological costs and benefits of symbiosis. To address this hypothesis, we (1) quantified EAs in seeds of 210 morning glory species from diverse, worldwide herbarium collections in order to determine the global distribution and diversity of Periglandula symbiosis, (2) obtained phylogenetically-informative ITS sequences from GenBank and determined the distribution of EA-positive (EA+) and EA− species across the host phylogeny to elucidate evolutionary history of the symbiosis, and (3) evaluated whether EAs and Periglandula symbiosis vary with plant life-history traits to understand potential selective benefits of symbiosis for the host. Our results provide insights into the evolution and chemistry of heritable fungal symbiosis, and the diversification of EAs in dicotyledonous plants.
Results
Distribution of EAs in morning glory species
One-quarter of morning glory species that we evaluated (53 of 210 species with mature seeds from herbarium specimens; Fig. 1) contained EAs in one or more samples, including 36 previously unreported species (Table 1, Supplementary Fig. 1, Supplementary Data 1). Considering that we were unable to obtain seed samples from herbarium specimens of many morning glory species, the total number of EA+ species is certainly larger. Ipomoea batatoides had the highest total mean ergot alkaloid concentration in seeds with I. hildebrandtii, I. kituiensis, I. racemosa, I. urbaniana, and three of four Stictocardia species all having greater than 1000 µg/g mean concentration (Supplementary Data 1). Cycloclavine, which was previously known only from one morning glory species (I. hildebrandtii), was detected in three species (I. cicatricosa, I. hildebrandtii and I. killipiana). The EAs chanoclavine, ergine, ergobalansine and ergonovine were widely distributed and detected in two-thirds or more of all EA+ species. We also found a mix of both positive and negative accessions for 13 of 19 EA+ species with three or more accessions, with six species consisting of only EA+ specimens (Supplementary Data 1). Species for which we had at least three accessions, and contained EAs in at least one sample, had alkaloids in 67% of the samples on average. Our results may underestimate the distribution and prevalence of EAs and Periglandula symbiosis given that a number of species were represented by only a single EA− sample (Supplementary Fig. 2a).
Global distribution of samples tested for EAs (n = 723). Gray circles represent herbarium specimens from Argyreia, Ipomoea, Stictocardia, and Turbina (now Ipomoea) species from the Global Biodiversity Information Facility83,84,85,86 to illustrate the geographical distribution of the tribe Ipomoeeae with higher concentrations of sample in darker areas.
In addition to seeds obtained from herbarium specimens, we sampled mature seeds from natural populations of two widespread and easily accessible EA+ species to investigate heterogeneity of EAs among populations. These collections revealed that 21 of 21 populations of I. leptophylla from the northern Great Plains region of the United States and 38 of 38 populations of I. pes-caprae from Florida beaches contained EAs, indicating that Periglandula symbiosis is ubiquitous in these regions. EA concentrations were significantly lower in I. pes-caprae (mean 12.6 μg/g) than in I. leptophylla (mean 28.2 μg/g; (t(115.4) = −8.62, p < 0.001). Unexpectedly, EA concentrations in I. pes-caprae were 43% higher in Gulf Coast populations compared to Atlantic Coast populations (t(36.0) = −3.91, p < 0.001), potentially reflecting higher nutrients in the Gulf of Mexico or genetic differentiation between the east and west sides of the Florida peninsula.
Latitude, sample age, and EAs
We tested whether latitude of origin and age of herbarium specimens affected the likelihood of detecting EAs. There was no significant effect of latitude on the presence of EAs (p = 0.46) or the total concentration of EAs among EA+ species (R2 = 0.004, p = 0.65). Herbarium specimens ranged between five and 179 years old, with the majority between 20 and 60 years old (Supplementary Fig. 2b), but we found that EA concentrations were not correlated with sample age (R2 = 0.005, p = 0.43, Supplementary Fig. 3), indicating that EAs persist in seeds over extended periods.
Comparison with previous survey
Eich5 previously categorized 98 morning glory species as “unambiguously positive”, “apparently devoid”, or having “conflicting reports” for EAs, based primarily on publications by other groups using variable methodologies and plant tissues. Our results from 68 of those 98 species agreed with the Eich classifications (Supplementary Data 1), but the majority of species represented in our seed samples were not included in Eich5. We identified four EA+ species listed as “apparently devoid” (I. adenioides, I. batatoides, I. graminea and I. mauritiana), although only one accession of each species contained EAs (N = 1, 3, 2, and 6 accessions, respectively). There were also eight Ipomoea species listed as EA+ that we found to be EA−, and we detected no alkaloids in 13 of 14 species listed as “contradictory”5. In the one other contradictory species, I. aquatica, we found that two of four accessions contained EAs. Polymorphism within species may contribute to some of these discrepancies, as may methodological differences among the numerous studies cited by Eich5 and potential misidentifications of species.
EAs distribution in host phylogeny
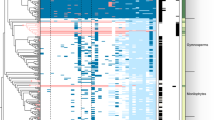
To evaluate the occurrence of EAs in relation to host phylogeny, we obtained published ITS sequences for 183 of our 210 sampled species, plus four outgroup species and 19 additional species reported by Eich5 (Supplementary Table 1, Supplementary Data 2). We found significant phylogenetic signal for the presence of EAs (Pagel’s λ = 0.8, D = 0.059, p < 0.001), suggesting that species with EAs are more closely related than expected by chance (Figs. 2, 3). By combining morning glory systematics25,26 with recent molecular results21,27, we found that most EA+ species occurred in four major clades, along with other single EA+ species occurrences dispersed through the phylogeny (Figs. 2, 3, Supplementary Figs. 4, 5). The largest clade of 22 species sampled here consists of three lineages: Argyreia, two ‘Turbina’ species, and the African clade Poliothamnus (ATP clade, Fig. 3; note that recent taxonomic treatments have dissolved the genus Turbina into Ipomoea28). It is well-represented in Asia but also includes African and South American distributions. The Pes-caprae clade (18 species) is largely New World but also includes Australian species and pantropical I. asarifolia, I. fimbriosepala, and I. pes-caprae. The other clades are Stictocardia (four species, largely African) and Tricolores (four species, the Americas). Phylogenetic analysis also suggests that EAs are absent from several large clades (Figs. 2, 3). For example, our samples from the Batatas clade, which includes sweetpotato, and the Jalapae clade, are entirely EA− (Fig. 3, Supplementary Fig. 4). A distinct heritable fungal symbiont that produces the toxic indolizidine alkaloid swainsonine occurs in I. carnea, I. costata and other species in the Jalapae and related clades29,30, potentially reflecting selection against redundant functions of multiple symbionts or their increased physiological costs such that only one endosymbiont can persist within a single host species31,32. In other clades, most species are EA- with occasional EA+ taxa (e.g., I. batatoides, I. mauritiana). Conversely, sporadic EA− species occur within the largely EA+ Pes-caprae and ATP clades (e.g., I. pandurata, A. capitiformis; Fig. 3). These instances suggest that both occasional gains and losses of symbiosis have occurred within clades. Overall, our results suggest that the Ipomoeeae common ancestor was not symbiotic, but rather that symbiosis arose independently in one or a few clades and diversified via vertical transmission and host speciation, with occasional host jumps through horizontal transmission by a yet unidentified mechanism, potentially through the transfer of epiphytic colonies (Supplementary Fig. 6).
Phylogenetic distribution of EA presence and seed mass. Species lacking seed mass data are represented by blank spaces. Pie charts at nodes indicate the relative likelihoods of the alkaloid ancestral character state. Black bars represent the origins of symbiosis. Lines and numbers indicate major clades of EA+ species: (1) Pes-caprae, (2) ATP, (3) Stictocardia, (4) Tricolores.
EAs chemistry and host phylogeny
For heritable symbionts lacking contagious spread, related host species should support related fungal symbionts with similar EA profiles through descent from common ancestors. Research with Epichloë-infected grasses indicates that the identities of EAs are determined by the fungus and not the host plant33. We detected nine EAs that occurred in 59 combinations (Table 1), and conducted phylogenetic principal component analysis (PCA) on the average concentrations of different alkaloids per species to determine whether EA profiles map onto host phylogeny. Analysis of similarity indicated significant dissimilarity of EA profiles among major clades (R = 0.31, p = 0.001; Fig. 4a, Supplementary Fig. 7a). For example, EAs in species from the Pes-caprae clade were dominated by ergobalansine while species in the ATP clade contained primarily ergosine (Table 1). Alkaloid profiles also differed significantly among species within the Pes-caprae clade (R = 0.52, p = 0.001, Fig. 4b) and within the ATP clade (R = 0.53, p = 0.001, Supplementary Fig. 7b).
Seed mass and EAs
We also measured seed mass from herbarium samples as a proxy for plant life history where larger-seeded species have lower growth rates and are longer lived than smaller-seeded species34. Seeds of EA+ species were significantly larger than seeds of EA− species (50.7 mg vs. 37.5 mg, t(118.1) = −3.98, p < 0.001, Fig. 5a), where seed mass varied 100-fold among species (Supplementary Data 1). To further explore this relationship, we transformed seed mass to a binary state and found a significant excess of large-seeded species containing EAs (X2 = 13.3, p < 0.001, Fig. 5b). We then performed Pagel’s test35, which revealed a significant correlation between seed mass and EAs after accounting for host phylogeny (p < 0.05, Fig. 3, Supplementary Fig. 8). Independent of EAs, there was a significant phylogenetic signal in seed mass (λ = 0.79, p < 0.001), suggesting that closely related species have more similar seed mass. Finally, we conducted phylogenetic regression36, which demonstrated that EA concentrations in EA+ species increased significantly with seed mass after accounting for phylogenetic correlation (p = 0.01, Fig. 5c). Thus, EA+ species have larger seeds than EA− species and EA concentrations increase with increasing seed mass in EA+ species.
Seed mass and EA concentrations are log10-transformed. a Number of species with (n = 53) or without (n = 157) EAs by mean seed mass. Stars along the x-axis indicate the mean seed mass of each group. Binomial regression line shows the probability of infection based on mean seed mass. b Proportion of small and large-seeded species with or without EAs. Dashed lines represent the expected number of EA+ species if the proportion of EA+ species is equivalent across seed mass. c Phylogenetic regression of seed mass and EA concentration in EA+ species.
Discussion
The potential origins of Periglandula symbiosis include horizontal transfer from Clavicipitaceae-infected grasses or from clavicipitaceous entomopathogens of hemipteran parasites of morning glories37,38. Notably, clavicipitaceous entomopathogens also exhibit diverse bioactive chemistry38. Phylogenetic studies clearly indicate that Periglandula is distinct from grass- and insect-infecting Clavicipitaceae24,39, supporting its ancient origins. Based on previous research21,40, we estimate the Periglandula symbiosis arose in the ATP clade c. 15 MYA. By comparison, Claviceps-infected grasses have been reported from Cretaceous amber 100 MYA41. Once established, there must have been occasional host shifts among clades of morning glories but wholesale horizontal spread is not supported by the phylogenetic patterns reported here. Phylogenetic patterns of heritable symbiosis also occur within other plant families. For example, fungal symbiosis and swainsonine production in locoweeds (Leguminosae) is limited to three host genera (out of 750), and Epichloë endophytes occur only in cool-season grasses in the subfamily Pooideae4.
What are the costs and benefits of EAs and Periglandula symbiosis? Heritable symbionts transmitted through seeds should be mutualistic given that they will not persist if they reduce host fitness42. While we do not have direct evidence that seed survival, germination, or resistance to predation are enhanced by EAs in morning glories, two experimental studies of Epichloë-infected grasses where EAs occur in seeds reported that seed-harvesting ants exhibit a significant preference for symbiont-free seeds over symbiont-infected seeds43,44. Similarly, multiple passerine bird species preferred symbiont-free grass seeds over symbiont-infected seeds45. Further, increased resistance to herbivory has been demonstrated in cool-season grasses symbiotic with Epichloë compared to symbiont-free conspecifics46, and pure EAs, including many found in morning glories, applied to food plants reduced insect growth47. In morning glories, EAs are concentrated in seeds where they could play a role against seed and seedling predators. For example, many morning glories are attacked by bruchine beetles48 (Chrysomelidae) where larvae feed within developing seeds. Poisoning of livestock following grazing on EA+ morning glory species has also been reported49. In I. tricolor, EAs in seeds are redistributed to host roots following germination50, and young EA+ plants are significantly more resistant to root-knot nematodes than EA− plants32. However, EA+ plants had reduced growth compared to EA− plants in the absence of nematodes, suggesting a physiological cost of symbiosis for host plants. Our results imply that EAs are disproportionately beneficial to species with larger seeds, which represent a larger investment by the maternal plant. This benefit may be direct where larger seeds and seedlings are subject to greater predator and pathogen pressure, or indirect where large seed mass is correlated with other plant life history traits like slower growth rate and longer life-span. More broadly, our results confirm the strong linkage between heritable fungal symbiosis and bioactive toxins across multiple plant families51, and suggest host-symbiont coevolution manifested as larger seeds with higher EA concentrations.
One limitation of this study is that we could only test 210 of 820 species in the Ipomoeeae because most herbarium samples did not contain mature seeds, given that specimens are typically collected during the diagnostic flowering stage. Extrapolation of our results to all morning glories suggests that there could be 150 or more additional EA+ host species yet to be detected. This may be a conservative estimate given that EA− species represented by one sample here could prove EA+ with additional samples (Supplementary Data 1, Supplementary Fig. 2a). Integrating our EA data into the larger dataset of Ipomoea species presented by Muñoz-Rodríguez et al.27. potentially identifies other likely EA+ species (Supplementary Data 3). For example, the Pes-caprae clade may contain other EA+ members such as I. procurrens and I. paludicola based on Muñoz-Rodríguez’s phylogeny. Independent of sampling depth, the ITS phylogeny could be expanded to a multigene phylogeny in the future with additional sequencing. At present, most of the available DNA sequence data for morning glories come from a single large published ITS phylogeny with over 400 species27. Another possible limitation arises if there are EA− Periglandula symbionts, which would underestimate symbiosis based on EA detection, although there is no evidence of this. For example, a previous study24 found that EAs and Periglandula sequences co-occurred in all eight morning glory species examined. Culturing of Periglandula has not yet been achieved, but would allow more detailed investigations of the symbiont, EA production, and host specificity.
Our results demonstrate that morning glories containing ergot alkaloids produced by Periglandula fungal symbionts are distributed worldwide and we report 36 previously unreported species containing EAs, suggesting that the numbers of host taxa engaged in Periglandula symbiosis will grow with additional research. Further, the EA+ species are concentrated in particular clades, consistent with the hypothesis that the Periglandula symbionts are vertically transmitted within clades with infrequent host jumps to distantly-related species. EA+ clades exhibited significant differences in EA profiles, reflecting genetic differences among Periglandula in EA biosynthetic pathways. EA+ species also had larger seeds with significantly higher EA concentrations, supporting the hypothesis that the symbiont provisions EAs to critical life-history stages for both the host and fungus. An important future direction will be to evaluate the importance of EAs and Periglandula symbiosis on host fitness in experimental or natural populations. Understanding why bioactive EAs and Periglandula symbiosis have diversified in certain clades and their relationship to plant life-history traits will provide a predictive framework that can be applied more broadly to other heritable fungal symbioses in plants.
Methods
Taxon sampling
We sampled mature seeds from a total of 210 morning glory species from 723 herbarium sheets, where species identity, mean alkaloid content, mean seed mass, and mean collection latitude are reported by species for each herbarium specimen (Supplementary Data 1, Supplementary Data 4). The sampled species included representatives from all continents except Europe (where we had only one sample; Fig. 1) and Antarctica, and from important genera, subgenera, and sections identified in various treatments of morning glories. They include species with localized distributions (e.g., Mexico, Somalia), regional distributions (e.g., South America, Tropical Africa), across regions (e.g., Africa and Asia) as well as pantropical distributions (Supplementary Data 1).
Herbarium specimens
We obtained permissions to sample mature seeds from herbarium specimens from the Missouri Botanical Garden (MOBOT), Vadense Herbarium (WAG, Netherlands) and the Australian National University Herbarium (ANU), in addition to seeds from personal collections of Convolvulaceae researchers and our own field collections in the United States. Based on the results from the Eich survey5, we focused our sampling on species in the tribe Ipomoeeae where all species previously reported to contain EAs occur. Our samples represented a subset of all potential species because most herbarium specimens examined did not have mature seeds. We recorded the most recently annotated name, determined mean seed mass (mg), estimated collection latitude based on location data on the herbarium sheet label and recorded the date of collection. We relied on the species determinations present on the herbarium specimen labels but realize that misidentifications are possible.
Field sampling of I. leptophylla and I. pes-caprae
In addition to herbarium specimens, we sampled natural populations of I. leptophylla in the northern Great Plains of the United States (Colorado, Nebraska, South Dakota and Wyoming). We identified plants along roadsides in public right-of-ways and collected mature fruits and seeds from 5 to 10 plants per sampling site. Populations of I. pes-caprae were sampled in Florida from beaches along both the Atlantic and Gulf coasts. Mature fruits and seeds were collected from extant plants or directly from the sand where dispersed seeds accumulate. For both species, seeds from a single site were combined into a single collection and one seed per population was then randomly selected for EA analysis unless it was less than the 20 mg needed for analysis, where additional seeds were combined until the threshold weight was reached.
Ergot alkaloid analysis
The EA content of seeds was determined by high performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS). Extracts were prepared by grinding the seeds into a fine powder using a Wiley mill. To prevent cross-contamination, we cleaned the Wiley mill between samples by vacuuming all parts, grinding millet seed (Pennisetum glaucum, previously determined to be devoid of EAs), vacuuming again and blowing all parts clean with compressed air. This process was previously tested using known alkaloid-positive samples and alkaloid-negative samples to confirm no detectable cross-contamination. The powdered seed material was then soaked in 1 mL methanol (99.93% A.C.S. HPLC Grade, Sigma-Aldrich) for three days at 4 °C with daily vortexing to promote extraction of EAs. EAs were identified and quantified by methods previously described50,52. In short, extracts were separated by reverse-phase HPLC on a C18 column (Prodigy 5-μm ODS3 [150 mm by 4.6 mm]; Phenomenex, Torrance, CA, USA) with a gradient of acetonitrile in aqueous ammonium acetate. Alkaloids were detected with dual fluorescence detectors set at excitation and emission wavelengths of 272 nm/372 nm and 310 nm/410 nm, respectively. Identity of individual peaks was confirmed by LC-MS as previously described53.
Based on the results of EA analysis, we classified species as EA+ if EAs were detected in any sample of that species, and as EA− if no EAs were detected in all samples of that species. Some species were included in our phylogeny where we did not have any samples, but were included in Eich5. We classified those species as EA+ or EA− following Eich.
Latitude, seed age, seed mass, and EAs
We tested for correlation between latitude and EA presence using a logistic regression. Correlation between latitude and EA concentration in EA+ species, as well as seed mass, was done using linear regressions. We used the absolute value of each species’ average latitude. EA concentrations and seed mass were averaged for each species. Supplementary Fig. 9 shows variation in seed mass for species with five or more samples. We tested whether EAs decay over time by regressing age on EA concentrations. Seed age was computed from the year 2021 for individual samples with a recorded year of collection (n = 135).
Estimating morning glory phylogeny with ITS sequence data
We downloaded the internal transcribed spacer region (ITS) sequence entries from GenBank available in 2020 for 183 out of 210 species that we sampled for EAs (no ITS sequence data were available for 27 of the sampled species). In addition, we added four Merremia species to represent outgroup taxa and 23 other important Ipomoeeae species (e.g., I. batatas, sweetpotato) previously reported in Eich5 (Supplementary Table 1). For each species, we obtained the complete ITS1 + 5.8 S rRNA + ITS2 sequence where possible. In some cases, this was obtained by combining partial sequences from multiple accessions of the same species. Relatively little sequence was missing based on comparing the partial sequences against the complete ITS sequences. In total, we obtained 206 sequences including four outgroup species. There were 175 complete ITS1 + 5.8S + ITS2 sequences, 10 sequences from combining partial sequences and 21 were partial ITS1 + 5.8S + ITS2 (Supplementary Data 2). These sequences were aligned using MAFFT v7.45054 using the L-INS-i alignment strategy. A maximum-likelihood phylogeny was then estimated using RAxML55 implemented in R package “ips” v0.0.1156 using the GTR + G + I model, rapid bootstrap analysis, random starting tree and 1000 replicates.
We used a combination of floras and treatments25,26,27,57, biogeography, and results from molecular systematics studies21,58,59 to identify 35 evolutionary lineages within Ipomoeeae (comprehensive list of references available upon request). This is not a formal classification but rather our best estimate of identifying clades that represent distinct groups of morning glories on independent evolutionary trajectories. Many of the evolutionary lineages were identified with high confidence given that they corresponded to traditionally identified taxa that could be circumscribed by diagnostic morphological characters and that received strong support as monophyletic groups from molecular systematic studies (e.g., Arborescentes, Batatas, Calonyction, Pharbitis, Poliothamnus)21,27. We also identified distinct lineages based on a combination of previous placements in traditional taxa and results from molecular systematic studies (American clades 1, 2, 3; Australian clade; Orthipomoea 1; Eriospermum; Erpipomoea 1, 2; Jalapae). These taxa deserve additional study. Finally, some clades identified here are based on the ITS phylogeny as well-supported monophyletic groups but unite morphologically diverse species from distinct traditional taxa (African clade 5, American clade 4).
We recognize the limitations of identifying distinct evolutionary lineages dependent on a single-gene tree, but 75% of our sampled species had no other gene sequences available. Our results are in general agreement with published multigene phylogenies of a smaller number of species, with some minor differences. For example, in our ITS phylogeny I. nil and I. hederacea are not sister species, although other evidence suggests that they are sister taxa and perhaps part of a species complex60. Similarly, two clades are resolved here that include the Leptophylla group, whereas in other studies they are united in a single clade consistent with their formal treatment based on morphology61.
Testing phylogenetic signal for ergot alkaloids and seed mass
Ergot alkaloid presence
We performed a test of phylogenetic signal to determine whether the distribution of EA+ species across the Ipomoeeae was random or clumped where closely-related species share the same EA status. We used the function “fitDiscrete” from the R package “geiger”62 that calculates the likelihood of observing trait values under a model63. We selected the ARD (all-rates-different) model as indicated by the lowest Akaike Information Criterion (AICc) score. We then used Pagel’s λ64 to assess the phylogenetic signal, which is a robust measure insensitive to taxon sample size and commonly used, facilitating comparisons with other investigations65. If λ is equal to or near 1, there is a strong phylogenetic signal. We created a null phylogeny by transforming our phylogeny so that there was no phylogenetic signal (λ = 0) and then compared the likelihood scores and computed the p-value of the two phylogenies to determine if our observed phylogeny significantly differed from the no-signal phylogeny.
Additionally, we computed Fritz and Purvis’s D66 (FPD) by using the function “phylo.d” from R package “caper”67. D-statistic values of a trait can be less than 1 (non-random distribution) or greater than 1 (random distribution).
Seed mass
The process for testing phylogenetic signal in seed mass was the same as for EAs except average seed weight for each species was first log10-transformed to normalize the data and the “fitContinuous” function from the R package “geiger”62 was used. The FPD statistic can only be applied to binary traits so we did not apply it to seed mass.
Ancestral character-state reconstruction
To assess the distribution and origins of EAs, ancestral character states were estimated using maximum likelihood methods for discrete characters64. Specifically, we reconstructed ancestral states for alkaloid presence (yes, no) and seed mass (large, small) using the R packages “ape”68,69 and “phytools”70. We used “ace” from R package “ape” to fit the best model of trait evolution for our data by comparing AICc scores which shows that ARD (all-rates-different) model was better than other models (equal-rates and symmetric). For each internal node the likelihood that the common ancestor was either EA+ or EA−, or whether the species had large or small seeds, was estimated using a joint estimation procedure68. Ancestors were designated as being one state or another if the likelihood of it being that state was greater than 75%.
We also carried out Bayesian stochastic character mapping for EAs71,72 via the function “make.simmap” using model “ARD” by running 1000 simulations and summarizing the results using “densityMap”70. From this analysis, we estimated for segments of the topology the probability that a particular portion of the lineage was EA+ or EA−. We integrated the results from both analyses to provide our best estimate of the pattern of ancestral character states across the phylogeny, as well as the number of independent origins of the symbiosis.
Correlated evolution in seed mass and alkaloid presence
To test for correlated evolution between seed mass and EAs in morning glory species while accounting for the confounding influence of shared ancestry, we used two methods. First, a phylogenetic regression was performed as implemented in the R package “phylolm”36 using log10-transformed seed mass data. Second, Pagel’s test of correlation35 was done as implemented in the R package “phytools”70 with the function “fitPagel”. Pagel’s correlation assesses correlated evolution between binary characters, so we therefore transformed seed mass into a binary trait. The average seed mass of each species was log10-transformed and the mean of these values was used to separate the large (>1.37, 23.4 mg) from the small (<1.37, 23.4 mg) group.
Intraspecific and interspecific differences in ergot alkaloid profiles
To evaluate differences in EA profiles between and within species, we used the “phyl.pca” function from the R package “phytools”70 to perform phylogenetically-corrected principal component analysis (PCA) for the full dataset of using averaged EA values for each species (Fig. 4a, Supplementary Fig. 7a). We used “vegan”73 to perform PCA for the Pes-caprae and ATP clades (Fig. 4b, Supplementary Fig. 7b) using all samples of each species. We did not correct for phylogeny in the clade-specific PCAs as we wanted to evaluate variation between multiple samples of species within the same clade. Due to the chemistry of EAs, only eight out of nine detected EAs were retained for the PCA and further grouped into six distinct EA chemotypes (colored boxes in Supplementary Fig. 1). Ergonovine, lysergic acid α-hydroxyethylamide (LAH), and ergine were combined as lysergic acid amides, while agroclavine was removed from the analysis as it is an intermediate alkaloid product (more details in Determining Ergot Alkaloid Chemotypes below). Significance of differences in EA profiles were calculated using analysis of similarity. All EA concentration measurements were transformed into relative concentrations for the analyses.
Determining ergot alkaloid chemotypes
Table 1 records all EAs quantified in the listed Convolvulaceae-Periglandula symbioses. Differences in EAs may be the result of different plant or fungus genotypes but also may result from environmental differences that were not controlled in the diverse herbarium collections sampled. While we present data on the nine EAs detected, we excluded certain EAs in our PCA analysis based on their position and significance in the biosynthetic pathway. Chanoclavine-I is an intermediate product transitional to other EAs except in I. graminea, where it was the sole EA detected. The presence of chanoclavine-I alone, indicates genetic differences where the pathway (Supplementary Fig. 1) downstream of chanoclavine-I is halted. Similar logic was applied in excluding agroclavine from PCA analyses. Simple amides of lysergic acid amides were considered as a group, because synthesis of individual lysergic acid amides is not completely independent of one another. While ergonovine can be synthesized independent of other lysergic acid amides74, synthesis of LAH is dependent on the genetic capacity to produce ergonovine. Ergine arises as a hydrolysis product from different derivatives of lysergic acid, in particular LAH75,76. Its accumulation may be affected by sample age and storage conditions, which were not controlled in the samples analyzed here. Therefore, simple amides of lysergic acid were considered as a single unit when classifying chemotypes. Unique end-products from branches off the clavine portion of the EA pathway, including cycloclavine and lysergol, were considered independent contributors to EA chemotype. Cycloclavine results from a unique combination of alleles in the EA pathway of certain fungi77. The biosynthetic origin of lysergol has not yet been determined, but its appearance is sporadic, and it does not serve as an intermediate in the EA pathway78. Two ergopeptine alkaloids, ergosine and ergobalansine, were detected in abundance, individually and in combination, in seeds from several EA+ species. Data from Claviceps purpurea indicate that different ergopeptine alkaloids result from allelic differences in the gene lpsA79. Small quantities of different ergopeptines also may result from permissive binding of alternate amino acid substrates by adenylation domains of peptide synthetases80,81.
Statistics and reproducibility
Statistical analyses used in this study are described in the Methods. Further information on the research design and reproducibility is available via the Nature Research Reporting Summary linked to this article.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data necessary to assess the conclusions of this study are available in the main text or the supplementary information. All Supplementary Data can be downloaded from Figshare (https://doi.org/10.6084/m9.figshare.14749512)82. All other data are available from the corresponding author on reasonable request.
References
Smith, D. C. & Douglas, A. E. The biology of symbiosis. (Edward Arnold Ltd., 1987).
Engl, T. et al. Evolutionary stability of antibiotic protection in a defensive symbiosis. Proc. Natl Acad. Sci. USA 115, E2020–E2029 (2018).
Zan, J. et al. A microbial factory for defensive kahalalides in a tripartite marine symbiosis. Science https://doi.org/10.1126/science.aaw6732 (2019).
Clay, K. & Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 160, S99–S127 (2002).
Eich, E. Solanaceae and Convolvulaceae: Secondary Metabolites. (Springer-Verlag Berlin Heidelberg, 2008).
Hofmann, A. & Tscherter, H. Isolierung von Lysergsäure-Alkaloiden aus der mexikanischen Zauberdroge Ololiuqui (Rivea corymbosa (L.) Hall. f.). Experientia 16, 414–414 (1960).
Schultes, R. E. Hallucinogens of plant origin. Science 163, 245–254 (1969).
Hofmann, A. Historical view on ergot alkaloids. Pharmacology 16, 1–11 (1978).
Florea, S., Panaccione, D. G. & Schardl, C. L. Ergot alkaloids of the family Clavicipitaceae. Phytopathology 107, 504–518 (2017).
Bush, L. P., Wilkinson, H. H. & Schardl, C. L. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 114, 1–7 (1997).
Miller, R. E., Buckley, T. R. & Manos, P. S. An examination of the monophyly of morning glory taxa using Bayesian phylogenetic inference. Syst. Biol. 51, 740–753 (2002).
Hallier, H. Versuch einer natürlichen Gliederung der Convolvulaceen auf morphologischer und anatomischer Grundlage. Bot. Jahrb. Syst. 16, 453–591 (1893).
McDonald, A. Origin and diversity of Mexican Convolvulaceae. Anales del Instituto de540 Biología. Serie Botánica 62, 65–82 (1991).
Baucom, R. S., Chang, S.-M., Kniskern, J. M., Rausher, M. D. & Stinchcombe, J. R. Morning glory as a powerful model in ecological genomics: tracing adaptation through both natural and artificial selection. Heredity 107, 377–385 (2011).
Kucht, S. et al. Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta 219, 619–625 (2004).
Steiner, U. et al. Molecular characterization of a seed transmitted clavicipitaceous fungus occurring on dicotyledoneous plants (Convolvulaceae). Planta 224, 533–544 (2006).
Ahimsa-Müller, M. A. et al. Clavicipitaceous fungi associated with ergoline alkaloid-containing Convolvulaceae. J. Nat. Prod. 70, 1955–1960 (2007).
Markert, A. et al. Biosynthesis and accumulation of ergoline alkaloids in a mutualistic association between Ipomoea asarifolia (Convolvulaceae) and a clavicipitalean fungus. Plant Physiol. 147, 296–305 (2008).
Steiner, U., Leibner, S., Schardl, C. L., Leuchtmann, A. & Leistner, E. Periglandula, a new fungal genus within the Clavicipitaceae and its association with Convolvulaceae. Mycologia 103, 1133–1145 (2011).
Stefanovic, S., Krueger, L. & Olmstead, R. G. Monophyly of the Convolvulaceae and circumscription of their major lineages based on DNA sequences of multiple chloroplast loci. Am. J. Bot. 89, 1510–1522 (2002).
Eserman, L. A., Tiley, G. P., Jarret, R. L., Leebens-Mack, J. H. & Miller, R. E. Phylogenetics and diversification of morning glories (tribe Ipomoeeae, Convolvulaceae) based on whole plastome sequences. Am. J. Bot. 101, 92–103 (2014).
Steiner, U. & Leistner, E. Ergoline alkaloids in convolvulaceous host plants originate from epibiotic clavicipitaceous fungi of the genus Periglandula. Fungal Ecol. 5, 316–321 (2012).
Schardl, C. L. et al. Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 9, e1003323 (2013).
Beaulieu, W. T., Panaccione, D. G., Ryan, K. L., Kaonongbua, W. & Clay, K. Phylogenetic and chemotypic diversity of Periglandula species in eight new morning glory hosts (Convolvulaceae). Mycologia 107, 667–678 (2015).
Verdcourt, B. Flora of tropical East Africa: Convolvulaceae (Crown agents for Overseas governments, 1962).
Austin, D. F. & Huáman, Z. A synopsis of Ipomoea (Convolvulaceae) in the Americas. Taxon 45, 3–38 (1996).
Muñoz-Rodríguez, P. et al. A taxonomic monograph of Ipomoea integrated across phylogenetic scales. Nat. Plants 5, 1136–1144 (2019).
Wilkin, P. A morphological cladistic analysis of the Ipomoeeae (Convolvulaceae). Kew Bull. 54, 853–876 (1999).
Cook, D. et al. Production of the alkaloid swainsonine by a fungal endosymbiont of the Ascomycete order Chaetothyriales in the host Ipomoea carnea. J. Agric. Food Chem. 61, 3797–3803 (2013).
Cook, D. et al. Biodiversity of Convolvulaceous species that contain ergot alkaloids, indole diterpene alkaloids, and swainsonine. Biochem. Syst. Ecol. 86, 103921 (2019).
Rossi, P. et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasit. Vectors 8, 278 (2015).
Durden, L., Wang, D., Panaccione, D. & Clay, K. Decreased root-knot nematode gall formation in roots of the morning glory Ipomoea tricolor symbiotic with ergot alkaloid-producing fungal Periglandula sp. J. Chem. Ecol. 45, 879–887 (2019).
Bouton, J. H. et al. Reinfection of tall fescue cultivars with non‐ergot alkaloid–producing endophytes. Agron. J. 94, 567–574 (2002).
Silvertown, J. W. Seed size, life span, and germination date as coadapted features of plant life history. Am. Nat. 118, 860–864 (1981).
Pagel, M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 255, 37–45 (1994).
Ho, L. S. T. & Ané, C. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408 (2014).
Kepler, R. M. et al. Host jumping onto close relatives and across kingdoms by Tyrannicordyceps (Clavicipitaceae) gen. nov. and Ustilaginoidea_(Clavicipitaceae). Am. J. Bot. 99, 552–561 (2012).
Boyce, G. R. et al. Psychoactive plant- and mushroom-associated alkaloids from two behavior modifying cicada pathogens. Fungal Ecol. 41, 147–164 (2019).
Píchová, K. et al. Evolutionary history of ergot with a new infrageneric classification (Hypocreales: Clavicipitaceae: Claviceps). Mol. Phylogenet. Evol. 123, 73–87 (2018).
Carruthers, T., Muñoz-Rodríguez, P. & Wood, J. R. I. & Scotland, R. W. The temporal dynamics of evolutionary diversification in. Ipomoea. Mol. Phylogenet. Evol. 146, 106768 (2020).
Poinar, G. J., Alderman, S. & Wunderlich, J. One hundred million year old ergot: psychotropic compounds in the Cretaceous. Palaeodiversity 8, 13–19 (2015).
Lipsitch, M., Siller, S. & Nowak, M. A. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 50, 1729–1741 (1996).
Knoch, T. R., Faeth, S. H. & Arnott, D. L. Endophytic fungi alter foraging and dispersal by desert seed-harvesting ants. Oecologia 95, 470–473 (1993).
Zhang, X. X., Li, C. J., Nan, Z. B. & Matthew, C. Neotyphodium endophyte increases Achnatherum inebrians (drunken horse grass) resistance to herbivores and seed predators. Weed Res. 52, 70–78 (2012).
Madej, C. W. & Clay, K. Avian seed preference and weight loss experiments: the effect of fungal endophyte-infected tall fescue seeds. Oecologia 88, 296–302 (1991).
Clay, K., Holah, J. & Rudgers, J. A. Herbivores cause a rapid increase in hereditary symbiosis and alter plant community composition. Proc. Natl Acad. Sci. USA 102, 12465–12470 (2005).
Kaur, N. et al. Mortality of potato psyllid (Hemiptera: Triozidae) on host clippings inoculated with ergot alkaloids. J. Econ. Entomol. 113, 2079–2085 (2020).
Janzen, D. H. Specificity of seed-attacking beetles in a Costa Rican deciduous forest. J. Ecol. 68, 929–952 (1980).
Gardiner, M. R., Royce, R. & Oldroyd, B. Ipomoea muelleri intoxication of sheep in Western Australia. Br. Vet. J. 121, 272–277 (1965).
Beaulieu, W. T. et al. Differential allocation of seed-borne ergot alkaloids during early ontogeny of morning glories (Convolvulaceae). J. Chem. Ecol. 39, 919–930 (2013).
Panaccione, D. G., Beaulieu, W. T. & Cook, D. Bioactive alkaloids in vertically transmitted fungal endophytes. Funct. Ecol. 28, 299–314 (2014).
Panaccione, D. G., Ryan, K. L., Schardl, C. L. & Florea, S. Analysis and modification of ergot alkaloid profiles in fungi. Methods Enzymol. 515, 267–290 (2012).
Ryan, K., Moore, C. & Panaccione, D. Partial reconstruction of the ergot alkaloid pathway by heterologous gene expression in Aspergillus nidulans. Toxins 5, 445–455 (2013).
Katoh, K., Rozewicki, J. & Yamada, K. D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform 20, 1160–1166 (2019).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Heibl, C., Cusimano, N. & Krah, F. S. ips: Interfaces to Phylogenetic Software in R. Available from CRAN at https://CRAN.R-project.org/package=ips (2014).
van Ooststroom, S. J. Convolvulaceae. in Flora Malesiana (ed. van Steenis, C. G. G. J.) 458–489 (Woltors-Nordhoff, 1953).
Miller, R. E., Rausher, M. D. & Manos, P. S. Phylogenetic systematics of Ipomoea (Convolvulaceae) based on ITS and waxy sequences. Syst. Bot. 24, 209–227 (1999).
Miller, R. E., McDonald, J. A. & Manos, P. S. Systematics of Ipomoea subgenus Quamoclit (Convolvulaceae) based on ITS sequence data and a Bayesian phylogenetic analysis. Am. J. Bot. 91, 1208–1218 (2004).
Eserman, L. A. Taxonomy and crossing relationships in a small group of morning glories (Ipomoea section Pharbitis). (Southeastern Louisiana University, 2012).
McDonald, J. A. Revision of Ipomoea section Leptocallis (Convolvulaceae). Harv. Pap. Bot. 1, 97–122 (1995).
Pennell, M. W. et al. geiger v2.0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics 30, 2216–2218 (2014).
FitzJohn, R. G., Maddison, W. P. & Otto, S. P. Estimating trait-dependent speciation and extinction rates from incompletely resolved phylogenies. Syst. Biol. 58, 595–611 (2009).
Pagel, M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 48, 612–622 (1999).
Münkemüller, T. et al. How to measure and test phylogenetic signal. Methods Ecol. Evol. 3, 743–756 (2012).
Fritz, S. A. & Purvis, A. Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 24, 1042–1051 (2010).
Orme, D. et al. The caper package: comparative analysis of phylogenetics and evolution in R. Available from CRAN at https://CRAN.R-project.org/package=caper (2013).
Paradis, E. & Schliep, K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019).
Paradis, E., Claude, J. & Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 20, 289–290 (2004).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Huelsenbeck, J. P., Nielsen, R. & Bollback, J. P. Stochastic mapping of morphological characters. Syst. Biol. 52, 131–158 (2003).
Bollback, J. P. SIMMAP: stochastic character mapping of discrete traits on phylogenies. BMC Bioinforma. 7, 88 (2006).
Oksanen, J. et al. Package vegan: community ecology package, version 2.0 10. Available from CRAN at https://CRAN.R-project.org/package=vegan (2013).
Ortel, I. & Keller, U. Combinatorial assembly of simple and complex D-lysergic acid alkaloid peptide classes in the ergot fungus Claviceps purpurea. J. Biol. Chem. 284, 6650–6660 (2009).
Flieger, M., Sedmera, P. & Vokoun, J. R̆ic̄icovā, A. & R̆ehác̆ek, Z. Separation of four isomers of lysergic acid α-hydroxyethylamide by liquid chromatography and their spectroscopic identification. J. Chromatogr. A 236, 441–452 (1982).
Leadmon, C. E. et al. Several Metarhizium species produce ergot alkaloids in a condition-specific manner. Appl. Environ. Microbiol. 86, e00373–20 (2020).
Jakubczyk, D. et al. Discovery and reconstitution of the cycloclavine biosynthetic pathway-enzymatic formation of a cyclopropyl group. Angew. Chem. Int. Ed. Engl. 54, 5117–5121 (2015).
Floss, H. G. Biosynthesis of ergot alkaloids and related compounds. Tetrahedron 32, 873–912 (1976).
Haarmann, T. et al. The ergot alkaloid gene cluster in Claviceps purpurea: extension of the cluster sequence and intra species evolution. Phytochemistry 66, 1312–1320 (2005).
Crawford, J. M., Portmann, C., Kontnik, R., Walsh, C. T. & Clardy, J. NRPS substrate promiscuity diversifies the xenematides. Org. Lett. 13, 5144–5147 (2011).
Fischbach, M. A., Lai, J. R., Roche, E. D., Walsh, C. T. & Liu, D. R. Directed evolution can rapidly improve the activity of chimeric assembly-line enzymes. Proc. Natl Acad. Sci. U. S. A. 104, 11951–11956 (2007).
Beaulieu, W. T. et al. Diversification of ergot alkaloids and heritable fungal symbionts in morning glories. figshare. https://doi.org/10.6084/m9.figshare.14749512 (2021).
GBIF.org. GBIF Occurrence Download, https://doi.org/10.15468/dl.cpsymh (2020).
GBIF.org. GBIF Occurrence Download, https://doi.org/10.15468/dl.89tm8m (2020).
GBIF.org. GBIF Occurrence Download, https://doi.org/10.15468/dl.qt635g (2020).
GBIF.org. GBIF Occurrence Download, https://doi.org/10.15468/dl.yu86zp (2020).
Acknowledgements
We thank Eric Knox (Indiana U.), James Solomon and George Yatskievtch (Missouri Botanical Garden), Brendan Lepschi and Kirsten Cowley (Australian National Herbarium) and Jan Wieringa (Vadense Herbarium, Wageningen U.) for help in obtaining seeds from herbarium specimens. We also thank Margaret S. Devall (USDA Center for Bottomlands Hardwood Research) and Kathleen Keeler (U. Nebraska-Lincoln) for seeds from their own collections. We also thank Richard E. Miller (Flower Diversity Institute, Arvada, CO) for his previous research on morning glory biology, valuable input on interpreting morning glory phylogeny and recommendations on our phylogenetic analyses. Further, we thank Pablo Muñoz-Rodríguez and Robert Scotland (Oxford University) for their extensive sequence database for, Ipomoea, which made our phylogenetic analyses possible. We thank Lauren Eserman, Natalie Christian, Michelle Afkhami, Mark Rausher, Erica Goss, Don Windsor, Stacey Smith, and Sunshine Van Bael for providing comments on the manuscript. Indiana U. students Michelle McKee, Eric Kimmel, Heather Smith and Clayton Tincher provided lab assistance. W.T.B. was supported by an Anne S. Chatham Fellowship in Medicinal Botany from the Garden Club of America, a grant-in-aid of research from Sigma Xi, an Ecological Society of Australia Student Research Grant and a travel grant from the American Philosophical Society Lewis and Clark Fund for Research and Field Exploration. Additional funding was provided by grant 2012-67013-19384 and project NC1183 from the United States Department of Agriculture, National Institute of Food and Agriculture to D.G.P. and grant 429440 from the Simons Foundation to the Smithsonian Tropical Research Institute to K.C. (W. Wcislo, P.I.).
Author information
Authors and Affiliations
Contributions
This paper represents part of W.T.B.’s PhD dissertation (2014) in the Department of Biology, Indiana U. W.T.B. and K.C. conceptualized the study. W.T.B., K.C., D.G.P., and K.L.R. collected and generated data. Q.N.Q. performed statistical analyses with input from K.C. K.C. led the writing and Q.N.Q. and D.G.P. contributed to writing, reviewing, and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Biology thanks Adrian Leuchtmann and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Caitlin Karniski.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beaulieu, W.T., Panaccione, D.G., Quach, Q.N. et al. Diversification of ergot alkaloids and heritable fungal symbionts in morning glories. Commun Biol 4, 1362 (2021). https://doi.org/10.1038/s42003-021-02870-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02870-z
This article is cited by
-
Plant-Microbial Symbioses in Coastal Systems: Their Ecological Importance and Role in Coastal Restoration
Estuaries and Coasts (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.