Abstract
Targeting reactive oxygen species (ROS) while maintaining cellular redox signaling is crucial in the development of redox medicine as the origin of several prevailing diseases including chronic kidney disease (CKD) is linked to ROS imbalance and associated mitochondrial dysfunction. Here, we have shown that a potential nanomedicine comprising of Mn3O4 nanoparticles duly functionalized with biocompatible ligand citrate (C-Mn3O4 NPs) can maintain cellular redox balance in an animal model of oxidative injury. We developed a cisplatin-induced CKD model in C57BL/6j mice with severe mitochondrial dysfunction and oxidative distress leading to the pathogenesis. Four weeks of treatment with C-Mn3O4 NPs restored renal function, preserved normal kidney architecture, ameliorated overexpression of pro-inflammatory cytokines, and arrested glomerulosclerosis and interstitial fibrosis. A detailed study involving human embryonic kidney (HEK 293) cells and isolated mitochondria from experimental animals revealed that the molecular mechanism behind the pharmacological action of the nanomedicine involves protection of structural and functional integrity of mitochondria from oxidative damage, subsequent reduction in intracellular ROS, and maintenance of cellular redox homeostasis. To the best of our knowledge, such studies that efficiently treated a multifaceted disease like CKD using a biocompatible redox nanomedicine are sparse in the literature. Successful clinical translation of this nanomedicine may open a new avenue in redox-mediated therapeutics of several other diseases (e.g., diabetic nephropathy, neurodegeneration, and cardiovascular disease) where oxidative distress plays a central role in pathogenesis.
Similar content being viewed by others
Introduction
Reactive oxygen species (ROS) have long been considered as an unwanted but inevitable byproduct of aerobic oxygen metabolism1. Excessive generation of ROS may lead to tissue damage and numerous undesired physiological consequences. Increased ROS level is linked to inflammation, aging, and pathogenesis of diseases like diabetes, cancer, atherosclerosis, chronic kidney disease (CKD), and neurodegeneration2,3,4,5. Recent understanding about the pivotal role of ROS as secondary messengers in cellular signaling to control processes like metabolism, energetics, cell survival, and death lead to a paradigm shift to the traditional “oxidants are bad—antioxidants are good” based simplistic view of redox biology6,7,8,9,10. Lack of attention towards the paradox between lethality of excessive intracellular ROS (oxidative distress) and the beneficial role of low concentration ROS (oxidative eustress) is the major underlying reason behind the failure of conventional antioxidant therapies using natural or synthetic antioxidants (e.g., α-tocopherol, ascorbic acid, β-carotene, curcumin, and numerous dietary polyphenols) that along with stoichiometric scavenging of intracellular free radicals, insulate redox signaling10,11,12. Moreover, meta-analyses of clinical trials show that the conventional antioxidants are not only ineffective, but also harmful, and even increase mortality12,13. The understanding that proper cell functioning critically requires a dynamic balance between oxidative eustress and distress (i.e., cellular redox homeostasis) forms the conceptual framework of redox medicine, a novel therapeutics that passivates the oxidative distress while maintaining the normal redox circuitry10,12,14,15,16. The cellular redox dynamics and its regulations, however, are still largely elusive because of the lack of effective pharmacological interventions17. In this regard, biocompatible transition metal oxide nanoparticles with potential electron-donating as well as accepting capability could be a viable option provided they are stable in the biological system, able to assimilate in the targeted tissue, and function in the physiological milieu.
Recently, we have shown that spinel structured citrate functionalized Mn3O4 nanoparticles (C-Mn3O4 NPs) have the unique ability to generate ROS in dark, and when injected into jaundiced animals can selectively degrade bilirubin (i.e., a toxic byproduct of heme metabolism) without showing adverse effects to other blood parameters18. At the same time, we found that the nanoparticles can catalytically scavenge free radicals particularly H2O2 in the in vitro reaction system. The microenvironment-controlled (i.e., presence of ROS, and subsequent changes in pH and dissolved O2) dynamic equilibrium between disproportionation and comproportionation involving surface Mn3+, Mn4+, and Mn2+ charge states present in the hausmannite structure of C-Mn3O4 NPs is responsible for such contrasting activity19,20,21. Therefore, depending upon the intracellular redox condition and pH (which can vary between intra- and extra-cellular environments, within the organelles and subsequently affect the redox activity of the nanoparticles), the nanoparticle has the potential to balance the oxidative distress and eustress, the most important feature of a redox medicine.
In this study, our major aim was to evaluate the potential of C-Mn3O4 NPs as a redox medicine against CKD. CKD, the progressive decline in kidney function, is one of the most serious global public health problem (with 8–16% worldwide prevalence) that originates from redox imbalance due to mitochondrial dysfunction and have no effective medication till date22,23,24,25,26. In order to understand the therapeutic potential of C-Mn3O4 NPs we used a cisplatin-induced C57BL/6j mice model of CKD. The mechanistic details of their pharmacological action in the maintenance of redox homeostasis and mitoprotection were further explored using cellular (human embryonic kidney cell, HEK 293) as well as animal model.
Results
Designing aqueous soluble C-Mn3O4 NPs to target kidney cells
The size, surface charge, and surface functionalization ligands determine the biodistribution of a nanomaterial inside living organisms. Protein corona (i.e., proteins adsorbed from plasma or intracellular fluids to the nanoparticle surface) is another important factor that critically influences the in vivo biodistribution and cellular internalization of nanoparticles27. Earlier studies have reported that particles with less than 8 nm diameters having moderate to high surface negative charge tend to accumulate in the renal system28. Therefore, care was taken at the time of synthesis to control the size of the Mn3O4 nanoparticles within the range of 6 nm. The transmission electron micrograph (TEM) of C-Mn3O4 NPs shows the monomodal distribution of nearly spherical particles with an average diameter of 5.58 ± 2.42 nm (Fig. 1a). High resolution (HR) TEM image of a single nanoparticle confirms the crystalline nature with clear atomic lattice fringe spacing of 0.311 ± 0.02 nm (Fig. 1a-inset) corresponding to the separation between (112) lattice planes of hausmannite Mn3O4 crystal. All x-ray diffraction (XRD) peaks corresponding to (101), (112), (200), (103), (211), (004), (220), (204), (105), (312), (303), (321), (224), and (400) planes of C-Mn3O4 NPs (Fig. 1b) exactly reflect the tetragonal hausmannite structure of Mn3O4 with a lattice constant of a = 5.76 Å and c = 9.47 Å and space group of I41/amd described in the literature (JCPDS No. 24-0734). The absence of any additional peak from other phases indicates the high purity of the synthesized material.
a TEM image of C-Mn3O4 NPs shows the spherical shape of the nanoparticles with the monomodal distribution. Inset shows an HRTEM image of a single nanoparticle having a high crystalline structure with 0.311 nm interfringe distance corresponding to the (112) plane. The other inset shows the histogram for the size distribution of the nanoparticles having an average diameter of 5.58 ± 2.42 nm. b Experimental XRD peaks of the nanoparticle exactly match that of Mn3O4 hausmannite defined in the literature (JCPDS No. 24-0734). c FTIR spectra of C-Mn3O4 NPs, Mn3O4 NPs, and citrate. Perturbation at Mn–O stretching at 413, 514, 630 cm−1 (shaded gray) of Mn3O4 NPs and carboxylic groups at 1066, 1112, 1410, 1619 cm−1 (shaded yellow) of citrate confirms strong covalent binding between citrate and the nanoparticle.
Surface functionalization with carboxyl rich ligand trisodium citrate not only made the nanoparticles biocompatible and aqueous soluble but also helped the surface charge to be negative (i.e., zeta potential, ξ = −12.23 ± 0.6 mV with electrophoretic mobility −0.96 ± 0.05 μ cm V−1 s). Fourier transformed infrared (FTIR) spectroscopy was used to confirm the binding of citrate to the surface of the nanomaterial (Fig. 1c). Broadening of the 630, 514, and 413 cm−1 bands associated with stretching vibrations of Mn–O and Mn–O–Mn bonds of Mn3O4 NPs along with substantial disruption of both symmetric (1410 cm−1) and asymmetric (1619 cm−1) stretching modes of carboxylates (COO−) of citrate indicates a strong covalent interaction between them.
Previously we showed that C-Mn3O4 NPs can selectively degrade bilirubin without affecting other blood parameters28. Here, initially, we evaluated their potential to scavenge H2O2 in an in vitro system using Rose Bengal (RB) degradation assay. RB has a distinct absorption peak at 540 nm. In the presence of H2O2, degradation of RB takes place causing a decrease in the 540 nm absorbance. When added to the reaction mixture, C-Mn3O4 NPs efficiently prevented the RB from H2O2 mediated degradation (Supplementary Fig. S1) indicating its strong radical scavenging potential towards H2O2.
C-Mn3O4 NPs maintain redox balance in HEK 293 cells against H2O2-induced oxidative distress
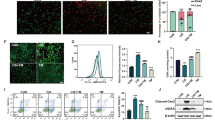
In order to test the ability of C-Mn3O4 NPs to combat oxidative stress in the cellular milieu, we used a cell-based approach. The HEK 293 cells pretreated with different concentrations of nanoparticles (3.75 to 60 μg mL−1) were exogenously exposed to H2O2 (100 µM) and cell viability was estimated using a well-known 2-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Fig. 2a). The survival rate for H2O2 treated cells was ~35% (p < 0.001 compared to control, one-way ANOVA, F(11, 48) = 136.7). The C-Mn3O4 NPs protected the cells from H2O2 induced cell death in a dose-dependent manner. Cell viability reached a maximum of ~85 and ~88% (p < 0.001 compared to H2O2 treated cells, One-way ANOVA, F(11, 48) = 136.7) in H2O2 exposed cells when pretreated with 30 and 60 μg mL−1 NPs, respectively. Pretreatment of the cells with similar concentrations of the NPs alone did not cause significant cellular mortality except the 60 µg mL−1 (~18%, p < 0.001 compared to control, one-way ANOVA, F(11, 48) = 136.7). Based on the results, we selected the 30 μg mL−1 C-Mn3O4 NPs for further experiments. Identical results were observed in the lactate dehydrogenase (LDH) assay (Fig. 2b). The presence of a high concentration of H2O2 inside the cell caused oxidative damage to the plasma membrane resulting in an increased release of LDH, a cytosolic enzyme, into the surrounding cell culture medium. Pretreatment with C-Mn3O4 NPs protected the cells from H2O2 induced oxidative damage resulting in a ~40% reduction in the LDH release (p < 0.001 compared to H2O2 treated cells, one-way ANOVA, F(3, 16) = 132.1). In post hoc analysis, the cells treated with C-Mn3O4 NPs alone also showed significant difference when compared to untreated control (p = 0.0057, one-way ANOVA, F(3, 16) = 132.1). However, it was not reflected in cell mortality. To evaluate the scavenging of H2O2 by C-Mn3O4 NPs under stress conditions, we monitored the intracellular oxidative stress using a ROS-sensitive fluorescence probe, dihydro dichloro-fluorescein diacetate (DCFH2-DA). DCFH2-DA is transported across the cell membrane and hydrolyzed by intracellular esterases to form nonfluorescent 2′,7′-dichlorofluorescein (DCFH), which is rapidly converted to highly fluorescent 2′,7′-dichlorofluorescein (DCF) in presence of ROS. The results illustrate that H2O2 exposure caused a substantial increase in the cellular ROS level indicated by the enhanced relative green fluorescence (λem/DCFH2-DA = 520 nm) intensity of DCFH2-DA (p < 0.001 compared to control, one-way ANOVA, F(3, 16) = 307.6) when measured using fluorescence microscopy (Fig. 2c, d) or flow cytometry (Fig. 2e). However, pretreatment with 30 μg mL−1 C-Mn3O4 NPs significantly lowered intracellular ROS level which was reflected in decreased fluorescence (~50% reduction; p < 0.001 compared to H2O2 treated cells, one-way ANOVA, F(3, 16) = 307.6) of the probe. The C-Mn3O4 NP treated cells also show a significant amount of ROS (p < 0.001 compared to H2O2 treated cells, one-way ANOVA, F(3, 16) = 307.6), which may be due to the inherent ability of the nanoparticles to generate ROS. The morphological observations in differential interference contrast (DIC) microscopy (Fig. 2d) support the results of cell viability and oxidative damage evaluation studies. The cells pretreated with C-Mn3O4 NPs prevented the shrinkage and congregation of the cell body due to H2O2 overexposure and maintained normal cellular architecture.
a Cell viability was measured using MTT. The gray shaded area represents H2O2 treatment. b LDH release. c Quantification of intracellular ROS as estimated from DCF fluorescence observed under confocal microscopy. d Confocal fluorescence micrographs of HEK 293 cells stained with DCFH2-DA and counterstained with DAPI. Cells were either left untreated or pretreated with C-Mn3O4 NPs (30 μg mL−1) prior exposure to H2O2 (100 µM). Scale bar: 30 µm. e Flow cytometry of HEK 293 cells stained with DCFH2-DA. Inset—intracellular ROS level quantified from flow cytometry analysis. f Dose-dependent internalization of C-Mn3O4 NPs in HEK 293 cells. The intracellular nanoparticle concentration as measured using inductively coupled plasma atomic emission spectroscopy (ICP-AES). g Correlation between biological impact (cell death and scavenging of ROS) and administered dose or intracellular nanoparticle content. All four nanoparticle concentration-biological response data are fitted with the Hill equation: \({{\mbox{y=}}}\,\,{{{\mbox{A}}}}_{1}{{\mbox{+}}}\frac{{{{\mbox{A}}}}_{2}{{\mbox{-}}}{{{\mbox{A}}}}_{1}}{1{{\mbox{+}}}{10}^{\left({\log }{{{\mbox{x}}}}_{0}{{\mbox{-x}}}\right){{\mbox{p}}}}}\). In bar plots data were expressed as Mean ± SD. In box plots, center lines show the medians; box limits indicate the 25th and 75th percentiles, whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles. Violins depict kernel density estimation of the underlying data distribution with the width of each violin scaled by the number of observations at that Y-value. Three lines (from the bottom to the top) in each violin plot show the location of the lower quartile (25th), the median, and the upper quartile (75th), respectively. The shaded area indicates the probability distribution of the variable. Individual data points are represented as colored circles (N = 5). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test was performed for comparison among the groups. The numbers inside the plots indicate numerical p values. p < 0.05 is considered significant.
The biological consequences of exposure to nanomaterials can only be understood in terms of content. Therefore, we evaluated the amount of nanoparticles internalized by the cells in terms of cellular manganese content using inductively coupled plasma atomic emission spectroscopy (ICP-AES). Figure 2f shows the dose-dependent uptake of C-Mn3O4 NPs in HEK 293 cells. The amount of intracellular manganese content (i.e., the nanoparticle content) increased logistically with the increase in the administered dose range of 3.75 to 60 µg mL−1, and reached a plateau at ~50 µg mL−1. Figure 2g depicts the correlation between the biological impacts (as measured by MTT and ROS assays) with both administered dose and intracellular nanoparticle content. Both prevention of cell death and quenching of intracellular ROS followed a logistic (Hill equation: \({{\mbox{y=}}}\,\,{{{\mbox{A}}}}_{1}{{\mbox{+}}}\frac{{{{\mbox{A}}}}_{2}{{\mbox{-}}}{{{\mbox{A}}}}_{1}}{1{{\mbox{+}}}{10}^{\left({\log }{{{\mbox{x}}}}_{0}{{\mbox{-x}}}\right){{\mbox{p}}}}}\)) relationship with administered dose and intracellular nanoparticle content.
C-Mn3O4 NPs prevent mitochondria, the master redox regulator, from H2O2-induced oxidative damage
Mitochondria despite being the primary source and regulator of intracellular ROS, are the most susceptible organelle to oxidative damage leading to redox imbalance and cell death29,30. So, to get further insight into the free radical scavenging activity of C-Mn3O4 NPs we evaluated their protective effect towards mitochondria. Treatment with H2O2 drastically decreased the mitochondrial membrane potential (ΔΨm), as indicated by enhanced rhodamine 123 (Rh123) fluorescence (Fig. 3a, b) along with a burst in mitochondrial ROS production, as indicated by the increased fluorescence of Mito-sox red (Fig. 3a, c). Pretreatment with 30 μg mL−1 C-Mn3O4 NPs significantly restored the ΔΨm and reduced the mitochondrial ROS. ΔΨm has a causal relationship with mitochondrial permeability transition pore (mPTP) opening. The results of the Ca2+ induced mitochondrial swelling assay indicated that the NPs were effective in preventing the H2O2 induced mPTP opening (Fig. 3d), therefore, maintaining mitochondrial integrity. The mitochondrial membrane depolarization and subsequent opening of mPTP led to a significant fall in the cellular ATP content (Fig. 3e). In C-Mn3O4 NP pretreated cells, such loss in ATP content was not observed. The opening of mPTP, fall in ΔΨm and ATP content cumulatively functions as a proapoptotic signal to initiate the cell death pathways, also reflected in the increased cytochrome-c oxidase activity (Fig. 3f). Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) constitute the intracellular antioxidant defense system that works in consort with mitochondria31,32,33. The accumulation of highly reactive oxygen radicals causes damage to biomolecules in cells and alters enzyme activities34,35,36. Hence, we extended our study towards evaluating the effect of H2O2 and C-Mn3O4 NPs in the ROS regulatory network. H2O2 exposure significantly reduced the activity of SOD, CAT, and GPx resulting in a decrease of the reducing pool of cellular thiol constituents (e.g., GSH) (Fig. 3g–j). Pretreatment with C-Mn3O4 NPs significantly attenuated the damage. In cells treated with C-Mn3O4 NPs alone, none of the detrimental effects were observed.
a Confocal fluorescence micrographs of HEK 293 cells stained with rhodamine 123, MitosoxTM red, and counterstained with DAPI. Cells were either left untreated or pretreated with C-Mn3O4 NPs (30 μg mL−1) prior exposure to H2O2 (100 µM). Scale bar: 10 µm. b Intensity of rhodamine 123 as a marker of mitochondrial membrane potential (Δψm). An increase in intensity indicates membrane depolarization. c Mitochondrial ROS level as quantified from MitosoxTM red fluorescence. d Change in Ca2+-induced mPTP opening. e ATP content. f Cytochrome c oxidase activity. g Superoxide dismutase (SOD) activity. h Catalase activity. i Glutathione peroxidase (GPx) activity. j Reduced glutathione (GSH) content. k Schematic representation of the redox homeostasis mechanism by C-Mn3O4 NPs against H2O2 distress through mitochondrial protection. In bar plots data were expressed as Mean ± SD. Violins depict kernel density estimation of the underlying data distribution with the width of each violin scaled by the number of observations at that Y-value. Three lines (from the bottom to the top) in each violin plot show the location of the lower quartile (25th), the median, and the upper quartile (75th), respectively. The shaded area indicates the probability distribution of the variable. Individual data points are represented as colored circles (N = 5). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test was performed for comparison among the groups. The numbers inside the plots indicate numerical p values. p < 0.05 is considered significant.
Thus, our cellular studies indicate that C-Mn3O4 NPs possess the distinctive property of scavenging intracellular ROS, inhibiting apoptotic trigger, preventing loss of antioxidant enzymes, and maintaining high cell viability by acting as a protector of mitochondria, the master regulator of cellular redox equilibrium (Fig. 3k schematically summarizes the whole sequence).
C-Mn3O4 NPs attenuate glomerular and tubulointerstitial damage in CKD mice
There is always a gap in the efficacies of a pharmacological agent tested between cellular and animal model. Limited bioavailability, nonspecific biodistribution, or unwanted metabolism often restricts the in vivo use of a cytoprotective agent37,38,39. Thus, we evaluated the potential of C-Mn3O4 NPs in the treatment of cisplatin-induced C57BL/6j mice, a well-established animal model for testing therapeutic interventions against CKD40,41,42. As depicted in Fig. 4a, treatment with C-Mn3O4 NPs alone did not cause any mortality during the experimental period. While, chronic administration of cisplatin resulted in significant mortality (~40% compared to control), administration of C-Mn3O4 NPs in the cisplatin-intoxicated group significantly reduced the mortality (Hazards Ratio, HR (Log-rank): 2.62; 95% CI of HR: 1.166–4.75; log-rank χ2 (Mantel–Cox): 5.23; df: 1; P = 0.0222) (Fig. 4a). The fourfold higher blood urea nitrogen (BUN) content (Fig. 4b), threefold higher GFR (Table 1), fourfold higher urinary albumin excretion (i.e., albuminuria) (Fig. 4c), and high urine albumin to creatinine ratio (ACR) (Table 1) along with significantly increased serum urea (Fig. 4d) and creatinine (Fig. 4e) illustrated induction of proteinuria and notable damage to the renal function of mice, the two hallmarks of CKD43,44,45. Treatment with C-Mn3O4 NPs (0.25 mg kg−1 body weight (BW)) to the cisplatin intoxicated animals considerably reduced BUN, GFR, urinary albumin, ACR, serum urea, and creatinine (Fig. 4b–e and Table 1). Treatment with citrate (the functionalization group) was unable to reduce any of the aforementioned parameters (Supplementary Fig. S2), confirming the observed effects solely due to the conjugated nanomaterial. This may be due to the low dose of citrate (i.e., 0.25 mg kg−1 body weight) used in the study. Such a low dose of citrate, a small molecule antioxidant having no catalytic activity, was not enough to prevent the oxidative damage caused by cisplatin. These results are well in agreement with the study by Kondo et. al.,46 where the authors revealed that citrate alone has no nephroprotective action, but it enhances the protective effect of bismuth subnitrate against the cis-diamminedichloroplatinum induced nephrotoxicity. Cisplatin intoxication caused weight loss in mice (Fig. 4f), suggestive of the systemic toxicity that frequently arises in individuals receiving this anticancer drug. Animals treated with C-Mn3O4 NPs were capable of mitigating weight loss. Next, we examined the external morphology of isolated kidneys from each group. The kidneys from the cisplatin exposed group deviated from the usual darkish brown to a pale brown color with a rough and uneven surface (Fig. 4g). The kidney to body weight ratio (i.e, kidney index) was also significantly higher (i.e., edema) in cisplatin-treated animals (2.1 ± 0.2 compared to 1.5 ± 0.1 mg g−1 of control, p < 0.001, one-way ANOVA, F(3, 28) = 31.7; Fig. 4h). Subsequent treatment with C-Mn3O4 NPs overturned the observed changes in morphology and kidney index.
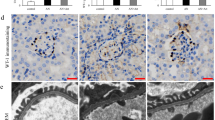
a Kaplan–Meier survival analysis curve. The darker shaded area represents the co-treatment period. b Blood urea nitrogen (BUN) content. c Urinary albumin excretion as an indicator of albuminuria, a hallmark of CKD. d Serum urea concentration. e Serum creatinine level. f Body weight at the end of the experimental period. g Photographs of kidneys incised after the experimental period. h Kidney index, defined as a kidney to body weight ratio (mg g−1). i Necrosis score as per the observation of an expert clinical pathologist. j Hematoxylin and eosin-stained liver sections. Insets show a magnified image of a single glomerulus. Red arrow: segmental glomerulosclerosis; Yellow arrow: global glomerulosclerosis; Yellow dotted region: mononuclear infiltration. Scale bar: 20 µm. k Glomerular injury score (GIS). l Tubular injury score (TIS). In bar plots data were expressed as Mean ± SD. Violins depict kernel density estimation of the underlying data distribution with the width of each violin scaled by the number of observations at that Y-value. Three lines (from the bottom to the top) in each violin plot show the location of the lower quartile (25th), the median, and the upper quartile (75th), respectively. The shaded area indicates the probability distribution of the variable. Individual data points are represented as colored circles or squares (N = 10). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test was performed for comparison among the groups. The numbers inside the plots indicate numerical p values. p < 0.05 is considered significant.
Hematoxylin and eosin-stained kidney sections of the control and C-Mn3O4 NP treated groups showed normal histologic features (Fig. 4j) with negligible necrosis score (Fig. 4i). The kidney sections from cisplatin intoxicated mice displayed several pathological features of CKD like focal segmental as well as global glomerulosclerosis along with interstitial fibrosis, diffused thickening of the capillary walls, glomerular hyalinosis, dilated or collapsed Bowman’s space, and glomerular retraction (Fig. 4j). Tubular atrophy, dilation of cortical tubules, increased mesangial matrix, obliteration of capillaries, necrosis, vacuolization, and interstitial mononuclear infiltration were the other features observed in this group. Treatment with C-Mn3O4 NPs notably reduced focal glomerular necrosis (Fig. 4j). However, sparse tubular changes like vacuolization, dilation, mild mononuclear infiltration, and detachment of epithelial cells were observed in this group. Overall, C-Mn3O4 NPs were able to efficiently revert the marked detrimental changes in the renal architecture of CKD animals. The histological observations are quantitatively reflected in the necrosis score (Fig. 4i), glomerular injury score (GIS; Fig. 4k), and tubular injury score (TIS; Fig. 4l).
Previous studies and our histological observations suggested an association between renal fibrosis and CKD47,48,49. So, we measured the renal hydroxyproline content, a byproduct of collagen metabolism, and biochemical marker of fibrosis. The results indicate almost a threefold increase in the hydroxyproline content (1.43 ± 0.07 compared to 0.51 ± 0.03 mg gm−1 tissue of control; p < 0.001, One-way ANOVA, F(3, 16) = 378.7) in the cisplatin intoxicated group (Table 1). In accordance with the histological findings, treatment with C-Mn3O4 NPs markedly reduced the hydroxyproline content (0.79 ± 0.06 mg gm−1 tissue; p < 0.001 compared to cisplatin-treated ones, One-way ANOVA, F(3, 16) = 378.7), suggesting a decrease in fibrotic damage (Table 1).
C-Mn3O4 NPs augment the intracellular antioxidant defense system
Oxidative stress proved to be one of the major causes of cisplatin-induced nephrotoxicity50,51,52. Therefore, we tried to ascertain whether C-Mn3O4 NPs contributed to nephroprotection by ameliorating oxidative stress. Signs of ROS-mediated damage including lipid peroxidation (in terms of thiobarbituric acid reactive substances, TBARS), reduction in cellular GSH pool, and inhibition of antioxidant enzyme activities were estimated. Exposure to cisplatin markedly increased the level of TBARS (Fig. 5a) and oxidative glutathione along with a reduction in GSH concentration. Furthermore, it inhibited the antioxidant actions of enzymes like SOD, CAT, and GPx (Fig. 5b–d). The results, in consensus with our cellular studies, indicate that C-Mn3O4 NPs rescued the renal cells from detrimental pleiotropic effects of increased ROS while maintaining the normal signaling circuitry.
a Extent of lipid peroxidation (MDA, malonaldehyde content) was measured in terms of thiobarbituric acid reactive substances (TBARS). b Superoxide dismutase (SOD) activity. c Catalase activity. d Glutathione peroxidase (GPx) activity. e Tumor necrosis factor-α level. f Interleukin-1β level. g Interleukin-6 level. h Immunohistochemical analysis of kidney tissues for detection of inflammatory damages. Macrophages are stained with anti-CD-68 antibodies (brown). Scale bar: 20 µm. The dotted circles indicate the regions with high CD68 positivity (i.e., macrophage infiltration). MDA, SOD, CAT, and GPx were estimated from kidney homogenate. TNF-β, IL-1β, and IL-6 were measured from serum. Violins depict kernel density estimation of the underlying data distribution with the width of each violin scaled by the number of observations at that Y-value. Three lines (from the bottom to the top) in each violin plot show the location of the lower quartile (25th), the median, and the upper quartile (75th), respectively. The shaded area indicates the probability distribution of the variable. Individual data points are represented as colored circles (N = 10). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test was performed for comparison among the groups. The numbers inside the plots indicate numerical p values. p < 0.05 is considered significant.
C-Mn3O4 NPs reduce renal inflammation
Macrophage infiltration in the kidney and subsequent rise in the plasma concentrations of pro-inflammatory cytokines like TNF-α are well-known features of CKD53,54,55. We found significant increases in plasma concentrations of TNF-α, IL-1β, and IL-6 in cisplatin-induced animals (Fig. 5e–g). Treatment with C-Mn3O4 NPs resulted in a notable decrease in cytokine levels. No difference was observed between the C-Mn3O4 NP treated and the control groups. Previous studies have demonstrated that direct adsorption of pro-inflammatory cytokines (e.g., TNF-α, ILs, etc.) by the nanoparticle surface can provide false positive or negative results about inflammatory responses56,57. Therefore, in order to further verify the findings of reduction in renal inflammation due to C-Mn3O4 NP treatment, we performed immunohistochemical (IHC) staining of the kidney sections with anti-CD68 antibodies, a well-known macrophage infiltration marker associated with the M1 macrophage phenotype. It is evident from Fig. 5h that the number of CD68 positive area is negligible in cisplatin+C-Mn3O4 NP co-treated animals compared to the cisplatin intoxicated animals, which showed pronounced staining of macrophage infiltrated area (marked in a dotted circle). The animals treated with C-Mn3O4 NPs alone showed characteristics similar to the control ones (Fig. 5h). Therefore, the results together suggest that the observed reduction in the inflammatory markers by the administered nanoparticles happened due to modulation of the inflammatory cascade, not by direct adsorption of ILs on the surface of the particle.
C-Mn3O4 NPs alleviate mitochondrial damage in CKD mice
Considering the inevitable role of mitochondria in the pathogenesis of CKD26,58,59,60,61,62,63 and the results of our in cellulo observations that C-Mn3O4 NPs protect mitochondria from H2O2 induced oxidative damage, we assessed the role of mitoprotection in the therapeutic efficacy of C-Mn3O4 NPs in animals. Ca2+-induced renal mPTP opening is one of the salient features of CKD26,64. Our data clearly show that the mitochondria isolated from the cisplatin intoxicated group were more sensitive towards Ca2+ manifested into a sharp decrease in 540 nm absorbance (Fig. 6a). Treatment with C-Mn3O4 NPs inhibited mPTP opening and maintained membrane integrity. ΔΨm and ATP content declined significantly as a result of cisplatin administration (Fig. 6b, c). These were accompanied by an increase in cytochrome c oxidase activity (Fig. 6d) and a reduction in dehydrogenase activity (Fig. 6e). The alterations were abrogated by C-Mn3O4 NP treatment. Mitochondrial parameters for the animals treated with C-Mn3O4 NPs alone showed no signs of toxicity and were analogous to the control animals. Thus, cisplatin-induced renal damage triggered the opening of mPTP, the decline in ΔΨm, and the induction of mitochondrial swelling that resulted in the release of cytochrome c in the cytosol leading to apoptosis. The ladder-like DNA fragmentation, a hallmark of apoptosis, was evident in the case of cisplatin-treated disease groups (Fig. 6f). Whereas, treatment with C-Mn3O4 NPs notably protected the mitochondria, inhibited cell death, and decreased the extent of DNA fragmentation. Figure 6g schematically illustrates the entire phenomena of redox-mediated nephroprotection by C-Mn3O4 NPs.
a Ca2+ induced mPTP opening measured by the decrease in 540 nm absorbance. b Mitochondrial membrane potential (Δψm) estimated using JC-1 fluorescence. c ATP content. d Cytochrome c oxidase (complex IV in the electron transport chain, ETC) activity in isolated mitochondria. e Succinate dehydrogenase (SDH, complex II in ETC) activity in isolated mitochondria. f DNA fragmentation level as a result of oxidative damage measured using agarose gel electrophoresis. In cisplatin-induced CKD animals DNA ladder formation, indicative of apoptotic DNA fragmentation, is clearly visible. The corresponding stacked bar plot shows the relative abundance of different-sized fragmented DNA in relation to the total DNA content of the lane. g Schematic overview of the comprehensive molecular mechanism of action of C-Mn3O4 NPs as a redox medicine against cisplatin-induced CKD. The numbers in the black circles indicate the sequence of events. In bar plots data were expressed as Mean ± SD. Violins depict kernel density estimation of the underlying data distribution with the width of each violin scaled by the number of observations at that Y-value. Three lines (from the bottom to the top) in each violin plot show the location of the lower quartile (25th), the median, and the upper quartile (75th), respectively. The shaded area indicates the probability distribution of the variable. Individual data points are represented as colored circles (N = 10). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test was performed for comparison among the groups. The numbers inside the plots indicate numerical p values. p < 0.05 is considered significant.
Pharmacokinetics and biocompatibility of C-Mn3O4 NPs
Over the years, pharmacokinetic (PK) studies have emerged as an integral part of drug development, especially for identifying the in vivo behavior of a drug. Therefore, we evaluated the time-dependent plasma concentration profile of C-Mn3O4 NPs in order to figure out how the nanoparticles are absorbed into and eliminated from the bloodstream. Intraperitoneal (i.p.) administration of C-Mn3O4 NPs (0.25 mg kg−1 BW) to mice generated the plasma Mn concentration vs. time profile displayed in Fig. 7a. The PK parameters were calculated using a non-compartmental approach, yielding a maximum plasma concentration (CMAX) of 3.1 ± 0.2 µg mL−1 at (tMAX) 2.0 ± 0.1 h. The plasma area under the curve (AUC) was 20.5 ± 1.8 µg h mL−1 with a Clearance of 12.2 ± 1.5 L h−1 kg−1. The mean plasma concentration curve for C-Mn3O4 NPs (Fig. 7a) presented a two-peak (at ~2.0 and ~12.0 h) absorption phase. The first peak is due to the absorption of NPs from the peritoneal cavity to the blood. While, intestinal absorption, variable gastric emptying, enterohepatic recirculation, and distribution, and reabsorption are possible interpretations for the second peak (at ~12.0 h)65. Next, we evaluated the time-dependent uptake and elimination profile of C-Mn3O4 NPs in the kidney up to 24 h (Fig. 7b). The manganese (i.e., C-Mn3O4 NP) content in the kidney increased exponentially reaching the maximum concentration of 2.28 ± 0.31 µg g−1 at ~10 h. The significantly increased concentration of nanoparticles in the kidney upon administration of C-Mn3O4 NPs clearly depicts its ability to enter the kidneys through the glomerular filtration barrier. Although the elimination phase started after 12 h, a sufficient quantity (0.95 ± 0.09 µg g−1) of nanoparticles remained in the kidneys even after 24 h post administration.
a Plasma concentration-time profile following intraperitoneal administration of C-Mn3O4 NPs as measured using inductively coupled plasma-atomic emission spectroscopy (ICP-AES). Inset shows the PK parameters calculated using a non-compartmental approach. The dotted line is a guide to the eye. b Time-dependent accumulation and elimination of C-Mn3O4 NPs in the kidney. Data were normalized to wet kidney weight. c Micrographs of hematoxylin and eosin-stained sections of different organs after 1 month of treatment with the therapeutic dose (0.25 mg kg−1 body weight, i.p.) of C-Mn3O4 NPs. Both control and treated animals maintained normal tissue architecture. Scale bars: liver-100 µm, brain-50 µm, ovary-20 µm, pancreas-50 µm, spleen-100 µm, testes-100 µm. Data were expressed as Mean ± SD. Individual data points are represented as white circles (N = 5).
Next, we investigated the effect of C-Mn3O4 NPs in other major organs in order to evaluate its biocompatibility. The hematoxylin and eosin-stained histopathological sections of the organs from C-Mn3O4 NP treated animals showed identical features to the organs collected from the control group (Fig. 7c). Livers of the nanoparticle-treated mice showed normal hepatic architecture with clearly detectable central vein, portal tracts, and hepatocytes arranged in cords with normal sinusoidal space. In the case of the pancreas, we found a normal pancreatic tissue structure comprising of pancreatic acini, pancreatic ducts, and β-cells islands. Nanoparticle treated spleen maintained ideal histological structure of white and red pulp. The testes of nanoparticle-treated animals did not show any remarkable structural changes. Their structure is comprised of well-organized seminiferous tubules and visible interstitial space. The maturation of spermatids were normal. The histological analysis also revealed normal ovarian structure with follicles at different growth phases and observable luteum follicle. The brain structure was also typical for C-Mn3O4 NP treated mice with normal cerebellum consisting of the granular and molecular layer, and visible Purkinje cells.
Discussion
In this study, we determined whether C-Mn3O4 NPs could be used as a redox medicine to treat CKD, an important clinical question considering the high prevalence of the disease and the nonavailability of effective medication. CKD is defined as the progressive and irreversible loss of renal function characterized by reduced glomerular filtration rate (GFR), increased urinary albumin excretion (albuminuria), or both43,45,66. Our results present evidence that treatment with C-Mn3O4 NPs significantly improved renal function, glomerular and tubulointerstitial injury, cellular antioxidant defense network in line with inhibition of pro-inflammatory immune response, and attenuation of mitochondrial dysfunction in response to the cisplatin toxicity. Our cellular and animal studies further enlightened the role of the unique mitoprotective as well as redox modulatory activity of C-Mn3O4 NPs in the therapeutic mechanism.
Several underlying factors played role in the selection of C-Mn3O4 NPs as the material of choice. These include the exciting redox modulatory properties (contrasting ability to function as prooxidant as well as catalytic antioxidant, the fundamental feature of a redox medicine), biodegradability, aqueous solubility, low cost of the nanomaterial along with apparent non-toxicity (permissible limit ~12 mg day−1), and abundance of manganese (Mn) as the catalytic metal centers or cofactors in several enzymes. Furthermore, previous reports67 about the nephroprotective action of catalytic antioxidants encouraged us to evaluate the therapeutic potential of C-Mn3O4 NPs against CKD. This study provides prima facie indication that other biodegradable/organic nanomaterials with sufficient mitoprotective and redox modulatory activity could be used to treat CKD, provided they can enter and stay in the kidney for sufficient time to depict therapeutic effect. In this regard, it is worth mentioning here that the bioavailability of a nano-compound to the kidney could be enhanced using efficient carrier molecules. For example, the peptide amphiphile micelles (PAM) functionalized with the zwitterionic peptide ligand, (KKEEE)3K, developed by Huang et. al.,68 have the ability to cross the glomerular filtration barrier for the efficient delivery of therapeutic agents to kidney.
Mitochondria have long been recognized for their canonical roles in cellular respiration and energy production26. Recently, they have emerged as the master regulator of a spectrum of molecular pathways including biosynthesis of macromolecules, maintenance of cellular redox equilibrium, calcium homeostasis, inflammation, and cell death69,70,71,72,73. Thus, mitochondria are poised to play a pivotal role in the functioning of the kidney, an organ with high energy demand, and rich in mitochondria, second only to the heart60. Our findings that C-Mn3O4 NPs maintain cellular redox homeostasis through the prevention of mPTP opening and ATP depletion discloses a key redox-mediated nephroprotective mechanism. Virtually, the renal proximal tubules are exclusively dependent on ATP generated by mitochondrial oxidative phosphorylation and are therefore vulnerable to oxidative distress due to mitochondrial damage50,74. Cisplatin accumulates in mitochondria and reduces the activity of all four respiratory complexes (I–V) involved in the electron transport chain (ETC), thereby a surge in mitochondrial ROS formation takes place along with mPTP opening, membrane depolarization, and impairment in ATP production, leading to cell death75,76. The cytotoxic mechanism of cisplatin essentially mimics the pathogenesis of CKD, thus an efficient reversal of damage in this rodent model is supposed to reflect the possible effects of a compound in higher animals. Data from our cellular as well as animal studies provide sufficient evidence that C-Mn3O4 NPs ameliorate mitochondrial ROS surge, prevent loss of membrane potential, inhibit mPTP opening, and stops ATP depletion, thereby prevents mitochondrial dysfunction, cellular redox imbalance, and tubular or glomerular cell death. As a result, the markers of CKD i.e., increased BUN, plasma creatinine, serum urea, and GFR returns to homeostatic condition.
This study provides a piece of direct evidence that C-Mn3O4 NPs can scavenge ROS, particularly H2O2 the longest living one in the cellular milieu. It also proves the ability of the nanoparticles in the prevention of mPTP from opening and subsequent maintenance of mitochondrial structure and function. However, it is not clear whether ROS scavenging protects mitochondria or protection of mitochondrial integrity results in ROS depletion. Several studies have shown that a compound having antioxidant properties cannot be a sustainable therapeutic solution to oxidative-stress-related disorders, fundamentally due to its inability to regenerate after a single reaction77,78,79. In contrast, redox nanomaterials have the potential to reduce oxidative stress either by autocatalysis or by protecting cellular machineries that control redox homeostasis, until the nanomaterials dissolute in the physiological milieu. Considering the causal relationship between mitochondria and cellular redox homeostasis and the efficacy of C-Mn3O4 NPs in the treatment of multifaceted diseases like CKD, we propose that both the mechanisms (i.e., ROS scavenging and mitochondrial protection) simultaneously take place.
The findings that C-Mn3O4 NPs can accelerate the revival of proximal tubule epithelium embodies a crucial nephroprotective function mediated by the nanoparticles. Kidneys show higher regenerative property following tubulointerstitial damage50. The proliferation of a subset of sublethally damaged, yet surviving, proximal tubule cells can contribute to the regenerative property of the kidney80. The acceleration of this process is sufficient to confer nephroprotection81,82. As revealed in our histological findings, the recovery rate of these cells (indicated by the structural integrity of the cellular architecture in hematoxylin and eosin-stained sections, and TIS scores) in C-Mn3O4 NP treated cisplatin exposed animals (Fig. 4j, k) was significantly higher and efficient than the auto-recovery (Fig. 4j, k). Several mechanisms can be proposed for the enhanced proliferation by C-Mn3O4 NPs. The restoration of structural and functional integrity of mitochondria and recovery of respiratory complexes may contribute towards the increased proliferation. It is well known that the mitochondrial ETC has a crucial role in cell proliferation through regulation of ATP generation, and supply of energy to proliferative pathways83,84. Earlier studies have demonstrated that mutations in ETC genes or the presence of ETC complex inhibitors cause a reduction in ATP synthesis, thus, obstructs progression through the cell cycle and proliferation85,86,87. Henceforth, it is reasonable to assume that the mitoprotective activity of C-Mn3O4 NPs have played a significant role in the revival of tubulointerstitial epithelial cells, in turn protecting the renal architecture. Additionally, ROS scavenging by C-Mn3O4 NPs may boost the proliferation because oxidative distress in proximal tubules causes cell cycle arrest and impedes cell-cycle progression28.
The favorable PK properties and biocompatible nature further indicates the possibility of using C-Mn3O4 NPs as a nano-drug. Beyond size, the localization of a nano-drug in the target organ allows assessment of its therapeutic regimen. The direct evidence that the nanoparticles enter the kidney crossing the glomerular filtration barrier and reside there for a sufficient amount of time further justifies our claim that the recovery is due to the therapeutic action of the nanoparticles. It is worth mentioning here that in virtually all in vivo studies using nanomaterials, the bulk of the material ends up in the spleen and the liver. The same is true for C-Mn3O4 NPs. In one of our recent studies, we revealed that C-Mn3O4 NPs also have a tendency to accumulate in the liver if orally administered for a longer (90 days) period of time88. Although, identifying the particular cell types that internalizes C-Mn3O4 NPs to the kidney or liver, and understanding the exact mechanisms of internalization are intriguing, they fall beyond the scope of this study and remain an attractive area for further investigation.
The role of intracellular redox regulation through mitoprotection in the therapeutic action of C-Mn3O4 NPs opens up further avenues for the treatment of several unmet diseases like diabetic nephropathy, neurodegeneration (e.g., Parkinson’s, Huntington’s, Alzheimer’s, multiple sclerosis), cardiovascular disorders, obesity, etc. where pathogenesis is very much dependent upon mitochondrial damage and associated redox imbalance89,90,91,92,93. Although in our study C-Mn3O4 NPs did not show any adverse effect, a detailed study on systemic toxicity, bio-distribution, PKs, and pharmacodynamics will greatly enhance the knowledge about its in vivo behavior. Furthermore, a detailed molecular study analyzing the genome and metabolome of C-Mn3O4 NP-treated animals may enlighten its ability to interfere in other pathogenesis pathways. As an outcome, the nano-drug could be repurposed for other therapies too.
Conclusion
There are very few published articles in contemporary literature that utilize the promising redox regulatory approach for the treatment of chronic diseases like CKD. On the other hand, several CKDs are reported to be due to redox imbalance in mitochondria. Our study suggests that C-Mn3O4 NPs could be an efficient redox medicine to attenuate renal injury and tubuleintestinal fibrosis as evidenced by the improved renal functions, reduction in biochemical markers of nephrotoxicity, reduced fibrotic content, and downregulated proinflammatory cytokines. The molecular mechanism involves regulation of the redox balance through synchronization of the causal relationship between mitoprotection and ROS scavenging by C-Mn3O4 NPs. The findings highly suggest the translational potential of C-Mn3O4 NPs as a redox nanomedicine for treating CKD in the clinic.
Methods
Synthesis of C-Mn3O4 NPs
A template or surfactant-free sol-gel method was followed for the synthesis of bulk Mn3O4 NPs at room temperature and pressure21. To functionalize the nanoparticles with ligand citrate, the as-prepared Mn3O4 NPs (~20 mg mL−1) were mixed extensively with citrate (Sigma, USA) solution (pH 7.0, 0.5 M) for 15 h in a cyclomixer. The time for mixing was carefully adjusted to have a nanomaterial in the size range of 4–6 nm. Non-functionalized larger NPs were removed using a syringe filter (0.22 μm).
Characterization techniques
TEM and HRTEM images were acquired using an FEI TecnaiTF-20 field emission HRTEM (OR, USA) operating at 200 kV. Sample preparation was done by drop-casting of aqueous C-Mn3O4 NP solution on 300-mesh amorphous carbon-coated copper grids (Sigma, USA) and allowed to dry overnight at room temperature. XRD patterns were obtained by employing a scanning rate of 0.02 s−1 in the 2θ range from 10 to 80 by a PANalytical XPERT PRO diffractometer (Malvern, UK) equipped with Cu-Kα radiation operating at 40 mA and 40 kV. FTIR (JASCO FTIR-6300, Japan) was used to confirm the covalent attachment of the citrate molecules with the Mn3O4 NPs. For FTIR studies, powdered samples were blended with KBr powder and pelletized. KBr pellets were used as a reference to make the background correction.
H2O2 scavenging by C-Mn3O4 NPs
The ability of C-Mn3O4 NPs to prevent H2O2 mediated degradation of sodium-containing dye RB (Sigma, USA) was used as an indicator of its H2O2 scavenging activity. The addition of H2O2 (10 mM) in the aqueous solution of RB (3.5 μM) leads to decolorization of the dye reflected in a decrease in absorbance (λmax = 540 nm). The presence of C-Mn3O4 NPs (50 μg mL−1) in the reaction mixture reduced degradation. All absorbance measurements were performed using Shimadzu UV-Vis 2600 spectrometer (Tokyo, Japan).
Culture of human embryonic kidney cells (HEK 293)
HEK 293 cells were maintained at 37 °C in 5% CO2 in RPMI 1640 growth medium (Himedia, India) that contained 10% fetal bovine serum (Invitrogen, USA), l-glutamine (2 mM), penicillin (100 units mL−1), and streptomycin (100 ng mL−1) (Sigma, USA). Before experimentation, the cells were washed twice and incubated with RPMI 1640 medium (FBS, 0.5%) for 1 h and then treated as described in the figure legends.
We used H2O2 (Merck, USA) to induce oxidative damage to the cells considering the fact that H2O2 is one of the most predominant intracellular ROS involved in cellular signaling as well as oxidative damage. While, several other compounds can endogenously produce of free radicals (i.e., CuSO4 or 3-amino-1,2,4-triazole), we did not use them in this study as none of them is naturally produced in the physiological system.
Measurement of cell viability
Cell viability was assessed by MTT and LDH assay. All cell lines were plated in 96-well plates at a density of 1 × 103 cells/well and cultured overnight at 37 °C. The treatments were performed as described in the figure legends. Next, MTT (5 mg mL−1; Himedia, India) was added to each well, with a final concentration of ~0.5 mg mL−1, and the cells were cultured for 4 h at 37 °C in a 5% CO2 atmosphere. The resultant purple formazan was dissolved by the addition of 10% sodium dodecyl sulfate (Sigma, USA) and the absorbance was read at 570 and 630 nm using a microplate reader (BioTek, USA). LDH release was analyzed using a colorimetric LDH cytotoxicity assay kit (Himedia, India) following the manufacturer’s instructions. Five independent experiments were performed in each case.
Measurement of intracellular ROS
The formation of intracellular ROS was measured with the DCFH2-DA method using both FACS and confocal microscopy. For FACS, cells were trypsinized, washed with 1X PBS, and stained with DCFH2-DA (15 μM; Sigma, USA) for 10 min at 30 °C in the dark after the treatments were completed. Ten thousand events were analyzed by flow cytometry (FACS Verse, Beckton Dickinson, SanJose, USA) and the respective mean fluorescence intensity (in FL1 channel, set with a 530/30 nm bandpass filter) values were correlated with the ROS levels. For confocal microscopy, 5000 cells were seeded and treated with agents described earlier. Post-treatment cells were stained with DCFH2-DA (5 μM) at 37 °C in dark and images were acquired with a confocal microscope (Olympus IX84, Japan). All parameters (pinhole, contrast, gain, and offset) were held constant for all sections in the same experiment.
Mitochondrial superoxide and ΔΨm detection
After treatment with the drug or vehicle, mitochondrial superoxide production was visualized using MitoSOXTM Red (Thermofisher, USA), a mitochondrial superoxide indicator. The ΔΨm in intact cells was assessed by confocal microscopy using Rh 123 (Sigma, USA). Cells were loaded with MitoSOXTM Red (0.5 μM) or Rh123 (1.5 μM) for 10 min at 37 °C and imaged using a confocal microscope (Olympus IX84, Japan). All parameters (pinhole, contrast, gain, and offset) were held constant for all sections in the same experiment. ImageJ (http://imagej.nih.gov/ij/) was used to quantify area normalized fluorescence intensities from the confocal images.
Animals and treatment
Healthy nondiabetic C57/6j mice of both sexes (8–10 weeks old, weighing 27 ± 2.3 g) were used in this work. Animals were maintained in standard, clean polypropylene cages (temperature 21 ± 1 °C; relative humidity 40–55%; 1:1 light and dark cycle). Water and standard laboratory pellet diet for mice (Saha Enterprise, Kolkata, India) were available ad libitum throughout the experimental period. All mice were allowed to acclimatize for 2 weeks before the treatment. The guideline of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India, was followed and the study was approved by the Institutional Animal Ethics Committee (Ethical Clearance No. - 05/S/UC-IAEC/01/2019).
The mice were randomly divided into five groups (n = 16 for each group): (1) control; (2) cisplatin; (3) cisplatin + C-Mn3O4 NPs; (4) C-Mn3O4 NPs; and (5) cisplatin + citrate. The experimental model of CKD was established according to the previous description. In brief, for induction of CKD, we used 8 mg kg−1 BW cisplatin (i.p.) in each alternative day for 28 days. After induction, we treated C-Mn3O4 NPs at 0.25 mg kg−1 BW (i.p.) for another 28 days. There was an overlap of 7 days between induction and treatment. Citrate (Sigma, USA) was used at a dose of 0.25 mg kg−1 BW for 28 days. All doses were finalized based on reported literature and pilot experimentation. As citrate treatment did not improve kidney function, data were not represented in the main manuscript.
Biochemical evaluations
Whole blood samples from treated mice were collected from retro-orbital sinus plexus and centrifuged at 2000 × g for 20 min to separate the serum. Urine samples were collected in metabolic cages during 24-h fasting conditions. Biochemical evaluations were performed using commercially available kits (Autospan Liquid Gold, Span Diagnostic Ltd., India) following the protocol described by respective manufacturers. GFR was estimated by the determination of urinary excretion of fluorescein-labeled inulin (FITC–inulin, Sigma, USA).
Histological examination
After incision, tissues from randomly selected mice were fixed with 4% paraformaldehyde, embedded in paraffin, and cut into a 5 μm thick section. After de-waxing and gradual hydration with ethanol (Merck, USA), the sections were stained with hematoxylin and eosin (SRL, India). The sections were then observed under an optical microscope (Olympus, Tokyo, Japan). It is noteworthy to mention here that the histopathologist was blinded to the treatment groups while scoring and evaluating the samples. For immunohistochemistry, kidney sections were incubated for 60 min with rat anti-mouse CD68 antibody (Santa Cruz Biotechnology, India) followed by a 30 min incubation with 10 mg mL−1 HRP-conjugated rabbit anti-rat secondary antibody (Santa Cruz Biotechnology, India). After detection of peroxidase activity with 3-amino-9-ethylcarbazole (Sigma, USA), sections were counterstained with Mayer’s hematoxylin (SRL, India).
Renal hydroxyproline measurement
For the measurement of renal hydroxyproline content, a previously described method was used32. In brief, snap-frozen kidney specimens (200 mg) were weighed, hydrolyzed in HCl (6 M; Merck, USA) for 12 h at 100 °C. Next, they were oxidized with Chloramine-T (SRL, India). Next, Ehrlich reagent (Sigma, USA) was added which resulted in the formation of a chromophore. Absorbance was measured at 550 nm. Data were normalized to kidney wet weight.
Renal homogenate preparation
Samples of kidney tissue were collected, homogenized in cold phosphate buffer (0.1 M; pH 7.4), and centrifuged at 10,000 rpm at 4 °C for 15 min. The supernatants were collected for further experimentation.
Assessment of lipid peroxidation and hepatic antioxidant status
The supernatants obtained in the previous stage were used to measure the activity of SOD, CAT, GPx, and GSH as well as the content of lipid peroxidation (MDA). Lipid peroxidation was determined in TBARS formation using a reported procedure31. SOD (Sigma, MO, USA), CAT (Abcam, Germany), and GPx activities (Sigma, MO, USA) were estimated using commercially available test kits following protocols recommended by respective manufacturers. The renal GSH level was determined by the method of Ellman with trivial modifications94.
Mitochondria isolation and mitochondrial function determination
Mitochondria were isolated from mouse kidneys following the method described by Graham95 with slight modifications. In brief, kidneys were excised and homogenized in a kidney homogenization medium containing 225 mM d-mannitol, 75 mM sucrose, 0.05 mM EDTA, 10 mM KCl, and 10 mM HEPES (pH 7.4). The homogenates were centrifuged at 600 × g for 15 min and the resulting supernatants were centrifuged at 8500 × g for 10 min. The pellets were washed thrice and resuspended in the same buffer. All procedures were performed at 4 °C.
Mitochondrial function was evaluated by determining ΔΨm using JC-1 (Sigma, MO, USA), ATP production (Abcam, Germany), and the activities of mitochondrial complexes succinate dehydrogenase and cytochrome c oxidase. mPTP opening was measured in terms of mitochondrial swelling by monitoring the decrease in absorbance at 540 nm after the addition of CaCl2 (100 mM).
Agarose gel electrophoresis for DNA fragmentation
Total renal DNA was isolated following a standard procedure96. DNA fragmentation was assessed using agarose gel electrophoresis. In a typical procedure, renal DNA (5.0 μg) was loaded on 1.5% agarose gel stained with ethidium bromide. Electrophoresis was carried out for 2 h at 90 V, and the resultant gel was photographed under UV transillumination (InGenius 3 gel documentation system, Syngene, MD, USA).
Pharmacokinetics, cellular and kidney uptake
For PK studies, animals were administered with C-Mn3O4 NPs (0.25 mg kg−1 BW; i.p.). Then, blood and kidney were collected at different time points, and Mn contents were estimated using ICP-AES (ARCOS-Simultaneous ICP Spectrometer, SPECTRO Analytical Instruments GmBH, Germany). The open acid digestion method was employed for the preparation of samples. In brief, dried tissue samples (in liquid nitrogen) were dissolved in a 3:2:1 mixture of HNO3 (Merck, Germany), H2SO4 (Merck, Germany), and H2O2 (Merck, Germany), and heated at 150 °C until only a residue remained. Then, the residues were diluted to 10 mL using Mili-Q water. Data were normalized to kidney wet weight.
For cellular internalization studies, HEK 293 cells were treated with different concentrations of C-Mn3O4 NPs and then subjected to ICP-AES following a similar procedure as described above. Data were normalized to the wet weight of the cells.
Statistics and reproducibility
Kaplan-Meier survival curves were used to illustrate mortality. Differences in survival between groups were assessed by the log-rank test with multiple pair-wise comparisons performed using the Mantel–Cox method. All quantitative data are expressed as Mean ± Standard Deviation (SD) unless otherwise stated. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test was performed for comparison between multiple groups. Beforehand, the normality of each parameter was checked by normal quantile–quantile plots. Sample size in our animal studies were determined following the standard sample sizes previously been used in similar experiments as per relevant literature. Designated sample size (in figure legends) always refers to biological replicates (independent animals). GraphPad Prism v8.0 (GraphPad Software) and Sigmaplot v14.0 (Systat Software, Inc.) were used for statistical analysis. Origin Pro 8.5 (OriginLab Corporation, MA, USA) was used for fitting the data. For all comparisons, a p value <0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All essential data are provided in the manuscript. The datasets generated and analyzed during this study to support the findings are available in a DOI-minting online open access repository, figshare, with the identifier (https://doi.org/10.6084/m9.figshare.14995122)97. Any remaining information can be obtained from the corresponding author upon reasonable request.
References
Wong, H. L. & Shimamoto, K. Sending ROS on a bullet train. Sci. Signal. 2, pe60 (2009).
Storz, P. Reactive oxygen species–mediated mitochondria-to-nucleus signaling: a key to aging and radical-caused diseases. Sci. STKE 2006, re3 (2006).
Carroll, B. et al. Oxidation of SQSTM1/p62 mediates the link between redox state and protein homeostasis. Nat. Commun. 9, 256 (2018).
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 (2000).
Ling, X. C. & Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replacement Ther. 4, 53 (2018).
Jones, D. P. Redefining oxidative stress. Antioxid. Redox Signal. 8, 1865–1879 (2006).
Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4, 180–183 (2015).
Saleme, B. et al. Tissue-specific regulation of p53 by PKM2 is redox dependent and provides a therapeutic target for anthracycline-induced cardiotoxicity. Sci. Transl. Med. 11, eaau8866 (2019).
Sies, H. Role of metabolic H2O2 generation: redox signalling and oxidative stress. J. Biol. Chem. 289, 8735–8741 (2014).
Casas, A. I. et al. Reactive oxygen-related diseases: therapeutic targets and emerging clinical indications. Antioxid. Redox Signal 23, 1171–1185 (2015).
Niki, E. Oxidative stress and antioxidants: distress or eustress? Free Radic. Biol. Med. 124, 564 (2018).
Elbatreek, M. H., Pachado, M. P., Cuadrado, A., Jandeleit-Dahm, K. & Schmidt, H. H. H. W. Reactive oxygen comes of age: mechanism-based therapy of diabetic end-organ damage. Trends Endocrinol. Metab. 30, 312–327 (2019).
Schmidt, H. et al. Antioxidants in translational medicine. Antioxid. Redox Signal. 23, 1130–1143 (2015).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
D’Autréaux, B. & Toledano, M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007).
Ursini, F., Maiorino, M. & Forman, H. J. Redox homeostasis: the golden mean of healthy living. Redox Biol. 8, 205–215 (2016).
Li, N. et al. Monitoring dynamic cellular redox homeostasis using fluorescence-switchable graphene quantum dots. ACS Nano 10, 11475–11482 (2016).
Polley, N. et al. Safe and symptomatic medicinal use of surface-functionalized Mn3O4 nanoparticles for hyperbilirubinemia treatment in mice. Nanomedicine 10, 2349–2363 (2015).
Takashima, T., Hashimoto, K. & Nakamura, R. Inhibition of charge disproportionation of MnO2 electrocatalysts for efficient water oxidation under neutral conditions. J. Am. Chem. Soc. 134, 18153–18156 (2012).
Takashima, T., Hashimoto, K. & Nakamura, R. Mechanisms of pH-dependent activity for water oxidation to molecular oxygen by MnO2 electrocatalysts. J. Am. Chem. Soc. 134, 1519–1527 (2012).
Giri, A. et al. Unprecedented catalytic activity of Mn3O4 nanoparticles: potential lead of a sustainable therapeutic agent for hyperbilirubinemia. RSC Adv. 4, 5075–5079 (2014).
Small, D. M., Coombes, J. S., Bennett, N., Johnson, D. W. & Gobe, G. C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 17, 311–321 (2012).
Beigrezaei, S. & Nasri, H. Oxidative stress in chronic kidney disease; an updated review on current concepts. J. Ren. Endocrinol. 3, e01–e01 (2017).
Dounousi, E. et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 48, 752–760 (2006).
Ruiz, S., Pergola, P. E., Zager, R. A. & Vaziri, N. D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 83, 1029–1041 (2013).
Galvan, D. L., Green, N. H. & Danesh, F. R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 92, 1051–1057 (2017).
Tekie, F. S. M. et al. Controlling evolution of protein corona: a prosperous approach to improve chitosan-based nanoparticle biodistribution and half-life. Sci. Rep. 10, 9664 (2020).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 8, 543–557 (2009).
Nissanka, N. & Moraes, C. T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 592, 728–742 (2018).
Musatov, A. & Robinson, N. C. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic. Res. 46, 1313–1326 (2012).
Adhikari, A. et al. Spectroscopic studies on dual role of natural flavonoids in detoxification of lead poisoning: bench-to-bedside preclinical trial. ACS Omega 3, 15975–15987 (2018).
Adhikari, A., Polley, N., Darbar, S., Bagchi, D. & Pal, S. K. Citrate functionalized Mn3O4 in nanotherapy of hepatic fibrosis by oral administration. Future Sci. OA 2, FSO146 (2016).
Adhikari, A. et al. Manganese neurotoxicity: nano-oxide compensates for ion-damage in mammals. Biomater. Sci. 7, 4491–4502 (2019).
Schieber, M. & Chandel Navdeep, S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Guachalla, L. M. & Rudolph, K. L. ROS induced DNA damage and checkpoint responses: influences on aging? Cell Cycle 9, 4058–4060 (2010).
Temple, M. D., Perrone, G. G. & Dawes, I. W. Complex cellular responses to reactive oxygen species. Trends Cell Biol. 15, 319–326 (2005).
Blomme, E. A. G., Yang, Y. & Waring, J. F. Use of toxicogenomics to understand mechanisms of drug-induced hepatotoxicity during drug discovery and development. Toxicol. Lett. 186, 22–31 (2009).
Li, A. P. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov. Today 6, 357–366 (2001).
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–716 (2004).
Shi, M. et al. Cisplatin nephrotoxicity as a model of chronic kidney disease. Lab. Investig. 98, 1105–1121 (2018).
Shah, S. V. & Rajapurkar, M. M. The role of labile iron in kidney disease and treatment with chelation. Hemoglobin 33, 378–385 (2009).
Landau, S. I. et al. Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int. 95, 797–814 (2019).
CKDP C. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375, 2073–2081 (2010).
El Nahas, A. M. & Bello, A. K. Chronic kidney disease: the global challenge. Lancet 365, 331–340 (2005).
Jha, V. et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013).
Kondo, Y. et al. Citrate enhances the protective effect of orally administered bismuth subnitrate against the nephrotoxicity ofcis-diamminedichloroplatinum. Cancer Chemother. Pharmacol. 53, 33–38 (2004).
He, J., Xu, Y., Koya, D. & Kanasaki, K. Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin. Exp. Nephrol. 17, 488–497 (2013).
Liu, N. et al. Suramin inhibits renal fibrosis in chronic kidney disease. J. Am. Soc. Nephrol. 22, 1064–1075 (2011).
Ninichuk, V. et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 70, 121–129 (2006).
Yu, X. et al. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-κB and improving mitochondrial function. EBioMedicine 36, 266–280 (2018).
Chirino, Y. I. & Pedraza-Chaverri, J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol. 61, 223–242 (2009).
Pabla, N. & Dong, Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73, 994–1007 (2008).
Lee, B. T. et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 16, 77 (2015).
Kim, H. W. et al. Effect of anti-tumor necrosis factor alpha treatment of rheumatoid arthritis and chronic kidney disease. Rheumatol. Int. 35, 727–734 (2015).
Amdur, R. L. et al. Inflammation and progression of CKD: the CRIC study. Clin. J. Am. Soc. Nephrol. 11, 1546–1556 (2016).
Brown, D. M., Dickson, C., Duncan, P., Al-Attili, F. & Stone, V. Interaction between nanoparticles and cytokine proteins: impact on protein and particle functionality. Nanotechnology 21, 215104 (2010).
Lee, Y.-G., Jeong, J., Raftis, J. & Cho, W.-S. Determination of adsorption affinity of nanoparticles for interleukin-8 secreted from A549 cells by in vitro cell-free and cell-based assays. J. Toxicol. Environ. Health 78, 185–195 (2015).
Forbes, J. M. & Thorburn, D. R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 14, 291–312 (2018).
Ishimoto, Y. & Inagi, R. Mitochondria: a therapeutic target in acute kidney injury. Nephrol. Dialysis Transplant. 31, 1062–1069 (2015).
Forbes, J. M. Mitochondria–power players in kidney function? Trends Endocrinol. Metab. 27, 441–442 (2016).
Bhargava, P. & Schnellmann, R. G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 13, 629–646 (2017).
Szeto, H. H. Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J. Am. Soc. Nephrol. 28, 2856–2865 (2017).
Szeto, H. H. et al. Mitochodria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J. Am. Soc. Nephrol. 22, 1041–1052 (2011).
Singh, D., Chander, V. & Chopra, K. Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology 207, 339–347 (2005).
Roels, H. et al. Relationship between external and internal parameters of exposure to manganese in workers from a manganese oxide and salt producing plant. Am. J. Ind. Med. 11, 297–305 (1987).
Lin, Y. F. et al. Resveratrol-loaded nanoparticles conjugated with kidney injury molecule-1 as a drug delivery system for potential use in chronic kidney disease. Nanomed. 12, 2741–2756 (2017).
Nishiyama, A. et al. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J. Am. Soc. Nephrol. 15, 306 (2004).
Huang, Y., Jiang, K., Zhang, X. & Chung, E. J. The effect of size, charge, and peptide ligand length on kidney targeting by small, organic nanoparticles. Bioeng. Transl. Med. 5, e10173 (2020).
Gulbins, E., Dreschers, S. & Bock, J. Role of mitochondria in apoptosis. Exp. Physiol. 88, 85–90 (2003).
Tait, S. W. G. & Green, D. R. Mitochondria and cell signalling. J. Cell Sci. 125, 807–815 (2012).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Ray, P. D., Huang, B.-W. & Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981–990 (2012).
Adhikari, A., Mondal, S., Darbar, S. & Pal, S. K. Role of nanomedicine in redox mediated healing at molecular level. Biomol. Concepts 10, 160–174 (2019).
Klein, K. L., Wang, M.-S., Torikai, S., Davidson, W. D. & Kurokawa, K. Substrate oxidation by isolated single nephron segments of the rat. Kidney Int. 20, 29–35 (1981).
Nowak, G. Y. Protein kinase C-α and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J. Biol. Chem. 277, 43377–43388 (2002).
Marullo, R. et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PloS ONE 8, e81162 (2013).
Warnholtz, A. & Münzel, T. Why do antioxidants fail to provide clinical benefit? Trials 1, 38 (2000).
Steinhubl, S. R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 101, S14–S19 (2008).
Persson, T., Popescu, B. O. & Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail? Oxid. Med. Cell. Longev. 2014, 427318 (2014).
Berger, K. & Moeller, M. J. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin. Nephrol. 34, 394–403 (2014).
Bagnis, C. et al. Erythropoietin enhances recovery after cisplatin‐induced acute renal failure in the rat. Nephrol. Dialysis Transplant. 16, 932–938 (2001).
Kawaida, K., Matsumoto, K., Shimazu, H. & Nakamura, T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc. Natl Acad. Sci. USA 91, 4357–4361 (1994).
Byun, H.-O., Kim, H. Y., Lim, J. J., Seo, Y.-H. & Yoon, G. Mitochondrial dysfunction by complex II inhibition delays overall cell cycle progression via reactive oxygen species production. J. Cell. Biochem. 104, 1747–1759 (2008).
Yoon, Y.-S., Byun, H.-O., Cho, H., Kim, B.-K. & Yoon, G. Complex II defect via down-regulation of iron-sulfur subunit induces mitochondrial dysfunction and cell cycle delay in iron chelation-induced senescence-associated growth arrest. J. Biol. Chem. 278, 51577–51586 (2003).
Mandal, S., Guptan, P., Owusu-Ansah, E. & Banerjee, U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev. Cell 9, 843–854 (2005).
Vohwinkel, C. U. et al. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 286, 37067–37076 (2011).
Merkwirth, C. et al. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 22, 476–488 (2008).
Adhikari, A. et al. Redox buffering capacity of nanomaterials as an index of ROS-based therapeutics and toxicity: a preclinical animal study. ACS Biomater. Sci. Eng. 7, 2475–2484 (2021).
Duchen, M. R. Roles of mitochondria in health and disease. Diabetes 53, S96–S102 (2004).
Lin, M. T. & Beal, M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006).
Johri, A. & Beal, M. F. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 342, 619–630 (2012).
Ritov, V. B. et al. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54, 8–14 (2005).
Ballinger, S. W. Mitochondrial dysfunction in cardiovascular disease. Free Radic. Biol. Med. 38, 1278–1295 (2005).
Ellman, G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77 (1959).
Graham, J. M. Isolation of Mitochondria, mitochondrial membrane, lysosome, peroxisome, and Golgi membranes from rat liver: Biomembrane Protocols I. Isolation and Analysis (eds. Graham, J.M. & Higgins, J.A.) 29–40 (Springer, 1993).
Petrik, J. et al. Apoptosis and oxidative stress induced by ochratoxin A in rat kidney. Arch. Toxicol. 77, 685–693 (2003).
Adhikari, A. et al. Associated data files for redox nanomedicine ameliorates chronic kidney disaese (CKD) by mitochondrial reconditioning in mice. figshare https://doi.org/10.6084/m6089.figshare.14995122 (2021).
Acknowledgements
M.D. thanks University Grants Commission (UGC), Govt. of India for Junior Research Fellowship. S.K.P. thanks the Indian National Academy of Engineering (INAE) for the Abdul Kalam Technology Innovation National Fellowship, INAE/121/AKF. The authors thank the DBT (WB)-BOOST scheme for the financial grant, 339/WBBDC/1P-2/2013. The authors would like to thank Sophisticated Analytical Instrument Facility SAIF), Indian Institute of Technology (IIT) – Bombay, India for carrying out the ICP-AES studies and Oncquest Laboratories Ltd., India for helping in IHC studies.
Author information
Authors and Affiliations
Contributions
A.A., M.B. and S.K.P. designed the experiments. A.A., S.M. and P.B. did synthesis, characterization, and in vitro studies. T.C. and M.B. performed cellular studies. A.A., S.D., S.M. and M.D. conducted animal experiments. A.K.D. performed the histological studies and analysis. R.G. performed the biodistribution, and cellular uptake studies during revision. A.A., H.A., J.T.A., A.S., S.A.A., M.B. and S.K.P. discussed and analyzed the results. A.A. wrote the manuscript and all authors contributed towards the writing of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Rob deLong and Anam Akhtar. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adhikari, A., Mondal, S., Chatterjee, T. et al. Redox nanomedicine ameliorates chronic kidney disease (CKD) by mitochondrial reconditioning in mice. Commun Biol 4, 1013 (2021). https://doi.org/10.1038/s42003-021-02546-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02546-8
This article is cited by
-
Chitosan functionalized Mn3O4 nanoparticles counteracts ulcerative colitis in mice through modulation of cellular redox state
Communications Biology (2023)
-
Chemoprevention of bilirubin encephalopathy with a nanoceutical agent
Pediatric Research (2023)
-
Nanoparticles in the diagnosis and treatment of vascular aging and related diseases
Signal Transduction and Targeted Therapy (2022)
-
Organ-specific therapeutic nanoparticles generates radiolucent reactive species for potential nanotheranostics using conventional X-ray technique in mammals
Applied Nanoscience (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.