Abstract
Species-rich plant communities can produce twice as much aboveground biomass as monocultures, but the mechanisms remain unresolved. We tested whether plant-soil feedbacks (PSFs) can help explain these biodiversity-productivity relationships. Using a 16-species, factorial field experiment we found that plants created soils that changed subsequent plant growth by 27% and that this effect increased over time. When incorporated into simulation models, these PSFs improved predictions of plant community growth and explained 14% of overyielding. Here we show quantitative, field-based evidence that diversity maintains productivity by suppressing plant disease. Though this effect alone was modest, it helps constrain the role of factors, such as niche partitioning, that have been difficult to quantify. This improved understanding of biodiversity-productivity relationships has implications for agriculture, biofuel production and conservation.
Similar content being viewed by others
Introduction
Plant productivity typically increases with species richness1,2,3. Efforts to understand this fundamental aspect of ecosystem function (i.e. overyielding) have understandably focused on mechanisms of overyielding, such as complementarity and selection effects4,5. Complementarity effects are often attributed to niche partitioning, which allows species-rich communities to capture more resources than species-poor communities6. Selection effects occur when more productive species are over-represented in species-rich relative to species-poor communities. However, niche complementarity and selection effects do not fully explain biodiversity-productivity relationships7,8,9. For example, while most plant communities overyield, some communities underyield and niche-partitioning and selection effects generally do not help explain this wide range of responses2.
Plant-soil interactions offer the potential to explain both overyielding and underyielding10,11,12. For example, species-specific soil pathogens can be expected to be more abundant and decrease plant growth more in monocultures than species-rich communities resulting in overyielding3,13,14,15. Conversely, species-specific soil mutualists can be expected to be more abundant and increase plant growth more in monocultures than species-rich communities resulting in underyielding16,17. Although it is near-impossible and likely inappropriate to individually characterize the effect of each species-specific soil pathogen and mutualist on plant productivity, it is possible to summarize the net effect of negative and positive plant-soil interactions using plant-soil feedback (i.e. PSF) experiments18. Thus, PSFs offer the potential to help explain both overyielding and underyielding in biodiversity-productivity relationships10,14.
Several experimental approaches have been used to explore the role of plant-soil interactions in biodiversity-productivity relationships5,14,19,20,21,22,23. Perhaps the best support comes from field and potted studies that used fungicide13 and microbial inoculations24 to demonstrate soil organism effects on biodiversity-productivity relationships, but these types of sterilization and inoculation experiments have been found to exaggerate PSF effects24,25. Several studies have used greenhouse experiments10,14,22,26, but greenhouse experiments have been found to produce PSFs that are not correlated with field-measured PSF27.
Two-phase, factorial field experiments remain the preferred approach for describing PSF28,29,30,31. In these experiments, plant species are grown on soils trained by other plant species. Due to the sample sizes required by factorial designs, these experiments have rarely been performed with more than a few species in the field19,32. We are not aware of any two-phase, field experiments that have tested the effects of PSF in biodiversity-productivity relationships.
Our goal was to test whether field-measured PSFs could help predict plant growth in experimental plant communities with 1–16 plant species. To do this, we measured PSFs in the field using a two-phase experiment in which each species was grown on soils trained by each other species in the experiment. This produced growth rates for each species on each soil training type that were used in plant-community simulation models to predict how much biomass each species would produce in a plant community. Plant communities with a range of species richnesses (1–16 species) were grown separately. Model prediction were compared to observed species biomass. To better understand how PSFs affected model predictions, the model was executed either with (PSF simulation model) or without (Null simulation model) PSF effects. To better understand the mechanisms determining how community biomass changes across species richness levels, we separated net biodiversity effects in each dataset (observed, Null, PSF) into complementarity and selection effect components11,33. Because this experiment produced 240 PSF values, it was also possible to test if PSF changed with phylogenetic distance which, if found, would help generalize PSF effects in the biodiversity-productivity relationship34,35.
Results
PSF experiment
The PSF experiment was performed, primarily, to produce plant growth rates on different soil training types to be used in plant-community simulation models, but we also report PSF index values because they are a common metric that provide a simple summary of plant-soil interactions29. PSF index values are the biomass on ‘self’ soils minus biomass on ‘other’ soils divided by the maximum of biomass on ‘self’ or ‘other’ soil. After a two-year training phase, plants created soils that changed subsequent plant growth by 27% (i.e. the mean absolute value of the PSF index was 0.27 in 2018). However, because most PSFs were negative but some were positive, the net effect of all PSFs was that plants created soils that decreased plant growth by 10% (i.e. a PSF index value of −0.10 in 2018). These effects increased over time during Phase II. The absolute value of the PSF index increased from 0.23 in 2017 to 0.27 in 2018 (T239 = −3.1, P = 0.002). The net value of PSF index values increased from 0.00 in 2017 to −0.10 in 2018 (T239 = 5.4, P < 0.001).
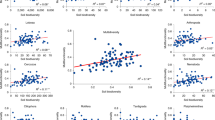
At the species*soil-level, 23 PSF index values were negative, and 13 were positive (i.e. 95% confidence interval did not overlap zero; Fig. 1a). These 36 PSF index values occurred across species so that 14 of 16 species demonstrated a PSF on at least one soil cultivation type (Fig. 1b). For conciseness, only 2018 species*soil-level PSF index values are shown in Fig. 1a. PSF index values among C3 grasses (T1,59 = 5.30, P < 0.001) and forbs (T1,59 = 3.65, P < 0.001) were negative, but PSF index values among C4 grasses (T1,59 = 0.65, P = 0.26) and legumes (T1,59 = 0.73, P = 0.23) were neutral. However, there was no difference among functional groups (F3,224 = 1.58, P = 0.19). There was also no correlation between species*soil-level PSF index values and phylogenetic distance (F1,239 = 0.01, P = 0.91).
PSF values from 2018 for each plant, on each soil-cultivation type shown in panel a. Orange and green highlighted values indicate negative and positive PSFs, respectively, and values with confidence intervals that do not overlap zero are bolded and underlined. Sample sizes derived from 27–35 replicates on ‘self’ soils and 5–9 replicates on each ‘other’ soil for each species. Exact sample sizes reported in Supplementary Table 1. Averaging across the 15 soil-cultivation type PSFs, produced one ‘species-level’ PSF value for each plant species (b). These species-level PSF values are shown for data from 2017 (grey) and 2018 (black). Each value represents the mean and standard error associated with the 15 soil-cultivation-specific PSF values. Asterisks indicate values that differed from zero in a one-sample t-test at (α = 0.05).
When species*soil-level PSF index values were averaged across soil cultivation types to produce one PSF index value for each species, there were five negative and three positive species-level PSF index values in 2017 and five negative and one positive species-level PSF index values in 2018 (Fig. 1b).
Biodiversity-productivity experiments
After 4 years, community biomass in the 2014 biodiversity-productivity experiment increased with species richness (Fig. 2; F1,59 = 36.4, P < 0.001) from 55.6 g m−2 in monocultures to 187.3 g m−2 in 16-species communities (Fig. 2). This 131.8 g m−2 difference represented a 237% increase in biomass production. Complementarity effects explained 172.5 g m−2 overyielding and selection effects explained 40.8 g m−2 underyielding in 16-species communities (Fig. 3a). Results were similar in the 1997 experiment where, after 4 years growth, biomass increased with species richness (F1,59 = 12.66, P < 0.001) from 78.5 g m−2 in monocultures to 183.4 g m−2 in 16-species communities (Fig. 2). This 104.8 g m−2 difference represented a 133% increase in biomass production. Complementarity effects explained 84.5 g m−2 overyielding and selection effects explained 10.5 g m−2 overyielding (Fig. 3b). Summarizing these two experiments, 16 species produced 118 g m−2 more than monoculture plots due to 129 g m−2 complementarity and −30.3 g m−2 selection effects.
Plant-community growth simulation models either with (PSF) or without (Null) plant-soil feedback effects predicted that biomass would increase with species richness (i.e. overyield). However, PSF simulation models correctly predicted this effect was caused by complementarity effects and Null models incorrectly predicted this effect was caused by selection effects (Fig. 3). The overyielding predicted by PSF simulation models represented 14% of the overyielding observed in the two biodiversity-productivity experiments. Each point represents total aboveground biomass in one community type (n = 55 or 63 for the 1997 and 2018 experiments, respectively). Large values from six outlier plots are not shown but were included in analyses. Lines represent the best-fit curves and shaded areas indicate the 95% confidence intervals. In each dataset, biomass increased with species richness (P < 0.05; see Results for details).
Data from experiments performed in 2014 and 1997 are shown in panels a and b, respectively. Data from simulation models that either included or excluded plant-soil feedback effects shown in panels c and d, respectively. Data from Net effects (black symbols) were separated mathematically into complementarity effects (red) and selection effects (blue). Plant-community simulation models that included plant-soil feedbacks correctly predicted that plant growth would increase with richness due to complementarity effects (c; red line) while the same models without plant-soil feedback effects incorrectly predicted positive selection effects (d; blue line) and negative complementarity effects (d; red line). Shaded areas indicate the 95% confidence intervals. Data from 63 (a) or 55 (b) different replicated plant communities. In each dataset, the net biodiversity effect increased with species richness (P < 0.05; see Results for details).
Model predictions
Plant-community simulation models that included a different growth rate for each soil training type (i.e. PSF simulation models) predicted that biomass would increase with species richness (F1,59 = 7.81, P = 0.007), from 60.1 g m−2 in monocultures to 76.1 g m−2 in 16-species communities (Fig. 2). This 16.0 g m−2 difference represented a 27% increase in biomass production and 14% of the overyielding observed in the two biodiversity-productivity experiments. Complementarity effects explained 15.0 g m−2 overyielding. Selection effects explained 1.0 g m−2 overyielding (Fig. 3c).
Null simulation models predicted that biomass would increase with species richness (F1,59 = 7.33, P = 0.009) from 60.7 g m−2 in monocultures to 70.7 g m−2 in 16-species communities (Fig. 2). This overyielding was caused by selection effects (11.8 g m−2) and not complementarity effects (−1.5 g m−2; Fig. 3d).
Both PSF and Null simulation model predictions were correlated with community biomass in the 2014 biodiversity-productivity experiment, though PSF simulation model predictions were closer to 1:1 (Observed biomass = 0.97 × PSF predicted biomass + 47.3) and had a stronger predictive ability (R2 = 0.20, P < 0.001, RMSE = 77.6) than Null simulation model predictions (Observed biomass = 0.81 × Null predicted biomass + 61.5, R² = 0.14, P = 0.003, RMSE = 81.6). Removing two outlier communities with large biomass further improved correlations and resulted in R2 values of 0.24 and 0.16 for PSF and Null simulation model predictions, respectively (Supplementary Fig. 1). Neither PSF nor Null simulation model predictions were correlated with biomasses from the 1997 biodiversity-productivity experiment (P > 0.05; Supplementary Fig. 1).
Discussion
PSFs improved understanding of the magnitude and mechanism of the biodiversity-productivity relationship. In experimental communities, plants grew 118 g m−2 more in diverse communities than in monocultures. This occurred because most plants grew better than expected from monocultures (i.e. complementarity effects) and not because dominant species were over-represented in communities (i.e. selection effects)11. PSFs helped explain this pattern because most plants created soils that decreased their own growth. Consequently, plants grew faster in communities, where they were surrounded by ‘other’ soils than in monocultures, where they were surrounded by ‘self’ soils10,13,24. This increased complementarity effects. Further, in Null simulation models, plants that grew most in monoculture were predicted to be over-represented in communities due to competition (i.e. selection effects). Negative PSF decreased these selection effects because dominant plants encounter higher proportions of ‘self’ soils than subdominant plants. The net effect of these changes was that PSF simulation models predicted 16.0 g m−2 more biomass in diverse communities than monocultures, due to complementarity effects. This 16.0 g m−2 represented 14% of the 118 g m−2 overyielding observed in experimental communities. PSF effects increased from 2017 to 2018 suggesting that PSF effects are likely to increase over time, though it is unlikely that PSFs would become a dominant determinant of overyielding. While 14% is a small portion of observed overyielding, results are important because they demonstrate diversity can increase productivity by suppressing plant disease. Results are also important because they help constrain the importance of other factors such as niche partitioning, which remain difficult to quantify23.
The magnitude and direction of PSFs in this study were broadly consistent with those from across the literature25,30,32,36, suggesting that PSFs likely play a similar role in other systems. The absolute value of PSFs (0.27) indicated that 2 years of plant growth created soils that changed subsequent plant growth by 27%. However, because PSFs were both positive and negative, the net PSF effect was smaller (i.e., a PSF value of −0.10 in 2018). Absolute PSF values reported across the literature tend to be larger (0.53)36, but are mostly measured in greenhouse conditions that are known to exaggerate PSF values27,36,37. This research and previous modeling efforts suggest a direct negative relationship between PSF and overyielding10. In other words, PSFs that decrease plant growth by 10% on ‘self’ soils are expected to produce 10% overyielding. Because PSFs often change plant growth by 10–50% and overyielding often changes plant growth by 100–200%, we expect that PSFs will often explain 5–50% of overyielding2,10,32,36.
PSF experiments are often performed by comparing plant growth on ‘self-trained’ soils to plant growth on soil trained by a non-specific mix of ‘other’ species29,31. At this species, or ‘mixed-other’ level, six species in 2018 realized significant PSFs. This ‘mixed-other’ approach has been criticized for overestimating PSF effects31. Our large factorial experiment allowed us to examine both ‘mixed-other’ and species*soil-level PSFs31,38. At the species*soil-level, 14 of 16 plant species realized a significant PSF on at least one soil type. While most species realized either positive or negative PSFs, three plant species demonstrated significantly positive PSFs on one soil type and significantly negative PSFs on a different soil type. For example, A. canescens PSF values ranged from −0.4 to +0.5. For this species, ‘self vs. other’ PSF experiments could be expected to report strongly positive, strongly negative or neutral PSF depending on the soil types used31. Variations in PSF, from positive to negative, were important for improving predictions of plant growth in communities. For example, positive PSFs helped improve correlations between predicted and observed community biomass by correctly decreasing the biomass of species with positive PSFs in communities (Supplementary Fig. 1)10. For example, a positive PSF for L. perennis on S. nutans soil, correctly resulted in less L. perennis biomass in L. perennis/S. nutans bicultures than predicted by the Null model. It should not be surprising that PSF vary as a function of the plant that trained a soil, but use of the factorial designs needed to demonstrate this pattern remain rare32. Thus, results provide a clear example of how broad species-level assessments of PSF can hide important soil-type-specific PSFs17,31,38.
PSFs in this and other studies tend to be negative, suggesting that plants accumulate species-specific pathogens. It is reasonable to expect that the negative effects of these pathogens would decrease with species relatedness, though evidence is mixed34,35. As the largest, factorial PSF experiment of which we are aware, this study provided a good opportunity to test for a phylogenetic effect, but we found no correlation between phylogenetic distance and PSF. This suggests that pathogen effects were highly species-specific and that phylogenetic distance beyond the population level may be inappropriate for generalizing PSF relationships39.
In previous biodiversity-productivity experiments, species richness explained 18–46% of variation in biomass among communities8,40,41. In our 2014 experiment, richness explained 12% of the variation in community biomass, suggesting large variability among communities, likely due to smaller plots and a shorter experiment duration. Despite this variability, our Null plant-community model explained 12% of the variation in plant species biomass and our PSF model improved this correlation to 20%. Removing data from two large outlier communities improved these correlations to explain 16 and 24% of variation (Null and PSF simulation model predictions, respectively). Although correlations were not large, results demonstrate that it is possible to predict species biomass in communities with similar accuracy reported for higher levels of organization (i.e. community biomass vs. community richness) that are generally assumed to be easier to describe42,43,44.
While promising in the short-term, our models were not correlated with plant-community composition in the 1997 experiment. Because factors from climate to anthropogenic nitrogen deposition to soil microbial community composition have likely changed in the 20 years between these two experiments, it is impossible to pinpoint why community biomass differs between the two experiments45. An implication of the poor correlation between the new and old data is that inference about the effects of PSF on plant-community development are likely to be time- or site-dependent46,47,48,49. However, despite a lack of correlation between predicted and observed biomass for specific communities, the general pattern of increasing aboveground biomass with species richness was consistent across both experiments and PSF simulation model predictions.
Conclusion
Biodiversity-productivity experiments were developed as a test of niche-partitioning effects, yet it remains difficult to quantify the extent to which niche partitioning determines biodiversity-productivity relationships7,50,51. Here, we report that PSFs explained 14% of the net biodiversity effect. Even though this effect is likely to increase over time, it is likely to remain modest relative to 100–200% increases in productivity across species richness treatments. Yet, demonstrating a 14% PSF effect is important because it quantifies how diversity can increase productivity in communities by suppressing plant disease. It is also important because it helps constrain the role of other factors (i.e. niche partitioning) in biodiversity-productivity relationships25,52,53. Future research that quantifies and integrates niche partitioning with PSF and other effects can be expected to improve predictions of the effects of species loss on plant-community productivity and resilience with implications for biofuel production and conservation6,54.
Methods
Two-phase PSF experiments have become the standard approach to measuring PSF, though they are typically performed in greenhouse conditions using plant monocultures, and their effects on plant growth in communities are rarely tested explicitly32. Here, we used a two-phase field experiment to measure the growth rate of each of 16 plant species on soils trained by each of the 16 species in the experiment (i.e. a factorial PSF experiments). The plant growth rates from this experiment allowed us to simulate the growth of any combination of species. We used these growth rates to simulate the growth of 63 unique plant communities that were grown separately. We then compare model predictions to observed plant growth. To better understand the mechanisms determining biodiversity-productivity relationships, we used a standard mathematical approach to parse the net change in community biomass with species richness into complementarity and selection effect components11.
Research was conducted in the Cedar Creek Ecosystem Science Reserve Long Term Ecological Research site, East Bethel, Minnesota, USA (45.403290 N, 93.187411 W). Previous research at the study site demonstrated large increases in community biomass with species richness (i.e. biodiversity-productivity relationships) that increase over time and are caused by complementarity55. Soils are sandy and of the Nymore series: mixed, frigid, Typic Udipsamment. During the four years of the study, mean annual precipitation and temperature were 723.0 mm and 6.5 °C, which is consistent with the 1963–2019 records at the site (769.3 mm and 6.6 °C, respectively).
We performed two experiments: a PSF experiment and a biodiversity-productivity experiment. Each experiment included 16 species used in an existing biodiversity-productivity experiment at the site (the Biodiversity II experiment; Table 1)40. Five species that together represented less than 3% of the biomass in the biodiversity-productivity experiment from 1997 (henceforth, BP1997) were excluded from our PSF and biodiversity-productivity experiments due to seed availability (Asclepias tuberosa L., Dalea villosa Nutt., Dalea candida Michx) and poor growth in previous experiments (Quercus macrocarpa Michx., Quercus ellipsoidalis E. J. Hill). Seeds were purchased from Prairie Moon Nursery (Minnesota, USA), Granite Seed (Utah, USA), Prairie Restorations Inc. (Minnesota, USA) and Minnesota Native Landscapes (Minnesota, USA).
In October 2014, a 1750-m2 fallow area adjacent to the BP1997 experiment was sprayed with a 5% glyphosate solution (Monsanto, Missouri, USA) and disc-harrowed to 15 cm to incorporate vegetation and homogenize soils. For the PSF experiment, 2720 plots (0.75 × 0.35 m) were established. For the biodiversity-productivity experiment, 232 plots (1.5 m by 1.5 m) were established. Plots were immediately adjacent to one another, but for all plots, a 35-cm deep by 4-cm wide trench was dug and lined with a root barrier to ensure that plant roots grew in target soil conditions (1-mm thick high-density polyethylene; Global Plastic Sheeting, California, USA). Throughout the PSF and biodiversity-productivity experiments, non-target plants were removed by hand several times each year.
PSF experiment
A two-phase, factorial PSF experiment was used28,29. Phase I began in April 2015. For each of the 16 target species, 10 g live seed m−2 was planted by hand in 170 replicate plots. During 2015, plots were watered weekly to promote establishment, and during the first 2 years plots were weeded once every 2 weeks to ensure the conditioned soils were monospecific. Seeded plant species grew in 2608 of the 2720 plots in Phase I. It is critical that Phase I plants do not re-establish from roots in Phase II because this growth would appear as a positive PSF. To prevent re-sprouting, vegetation was killed with a 5% glyphosate treatment and aboveground biomass removed, plots were then hand-tilled with a garden claw (~75% of plots) or rototiller as necessary (~25% of plots; Stihl Inc., Delaware, USA), November 2016. Finally, plots were again treated with herbicide in April 2017, prior to Phase II seeding, which replicated Phase I seeding rates. Glyphosate application may affect mycorrhization and therefore decrease positive PSF56, but it was critical to ensure that all Phase I plants were killed because re-sprouting plants have the potential to create large, false positive PSFs.
For Phase II, each target species was to be planted in 35 replicate plots with ‘self’ soils and nine replicated plots with each of the 15 ‘other’ soils. Because some target species failed to establish in Phase I, actual replication ranged from 27 to 35 replicates on ‘self’ soils and five to nine replicates on each ‘other’ soil (Supplementary Table 1). Further, each target species was randomly assigned to five to nine replicate plots that had no Phase I growth. These ‘control’ plots had no plant growth during Phase I and were used to parameterize one of the Null models. During Phase II, plots were weeded once per month.
Plant cover in every plot was assessed by visual estimation in August 2017 and September 2018 and plant aboveground biomass was clipped, dried and weighed in October 2018. The 2017 percent cover data was converted to biomass values using the 2018 percent cover to biomass relationship.
Biodiversity-productivity experiment
In April 2015, 63 plant communities containing 1–16 plant species were planted in 232 plots. Plant communities with 1, 2, 4, 8, 14, and 16 species were established with 16, 14, 9, 9, 14, and 1 unique community compositions for each richness level, respectively (Supplementary Data 1). Each unique community composition was planted in three replicate plots, except monocultures which were each planted in four replicates plots, and 16-species communities which were planted in 30 replicate plots. Community compositions were designed to replicate those in the BP1997 experiment (Supplementary Data 1)55,57. For 40 of 63 communities, species composition in the new and existing experiments were identical. The remaining 23 communities differed in that they did not include the five species described above, but again, these species represent less than 3% total biomass in BP1997.
Each plot received 10 g live seed m−2, with each seeded species in the community representing equal proportions of the seed mix. Plots were watered in the first year of the study (2015) and were weeded every two weeks for the first 2 years of the study. Thereafter, plots were weeded once per month. In August 2017, percent plant cover by species was assessed by visual estimation to the nearest percent. Rather than removing thatch by burning (as in BP1997), total biomass was harvested and removed to prevent melting the plastic root barrier. In August 2018, plant cover in each plot was assessed by visual estimation, then randomly selected 15 cm by 150 cm strips were clipped, sorted to species, dried to constant weight at 60 °C and weighed to the nearest 0.1 g. The remaining biomass was then clipped, dried and weighed. Percent cover to dry biomass correlations were used to transform percent cover values to biomass values.
To provide an additional test of the role of PSF in the BP relationship, we also used published data from the fourth year of the BP1997 experiment (https://www.cedarcreek.umn.edu/research/data). Cover to biomass relationships reported for 2007 were used to convert species-level cover data to species-level biomass that were then scaled to match observed community biomass40.
Statistics and reproducibility
Calculating and testing PSF values
PSF index values were calculated from aboveground biomass data as follows: PSF = (S-O)/max(S,O) where S is the aboveground biomass produced in Phase II on ‘self’ soils, O is the aboveground biomass produced in Phase II on ‘other’ soils, and max(S,O) selects the larger of S and O. This calculation and the commonly used log response ratio have been found to be superior to other calculations, but the calculation we use has the added benefit that it produces values that are bound by −1 and 1 and are easily interpretable as the proportion change in growth among soil types29. The mean and error associated with these values was estimated using bootstrapped confidence intervals calculated using the sample_n command from the R package ‘dplyr’. Because PSFs were measured for 16 species on 15 soil types, analyses yielded 240 species*soil-level PSF values. Because the mean of large positive and large negative PSF values can be zero, and therefore ‘mask’ PSF effects, we also calculated the absolute value of PSF values.
The 240 species*soil-level PSF values were considered positive or negative when their 95% confidence interval did not overlap zero. Variation in species*soil PSF values is derived from the 27–35 replicate “self” and 5–9 replicate “other” field plots. Species-level PSF values were then calculated as the mean PSF value across 15 soil training types. Variation in species-level PSF is derived from the 15 soil types. To determine if species-level PSF values differed from zero, one-way t-tests were used. Species-level PSF were considered different from zero when P < 0.05. To test whether or not PSF values changed between the first and second year of Phase II, a one-way ANOVA with year as a factor was used (‘aov’ and ‘TukeyHSD’ in R programming). Differences among years were considered significant when P < 0.05. Differences among functional groups were tested with a t-test and effects of phylogenetic distance were tested using a correlation between phylogenetic distance and species*soil-level PSF values58.
Plant-community growth simulation models
PSF experiments describe plant growth on soils trained by different species, but do not describe how plants grow in communities. To assess how these PSFs are likely to affect plant growth in communities, we use plant-community simulation models with and without PSF effects to predict plant biomass and we compare model predictions to plant biomass observed in experimental plant communities. Broadly, these models allow each plant in a community to grow from seed at rates determined from the PSF experiment. Plant growth is eventually limited by a carrying capacity. The best-performing discrete plant-community simulation models in a similar previous study were used (i.e. the ‘logistic species-level-K model’ and the ‘logistic constant-K model’)59,60. In this logistic growth simulation model, species-conditioned soils ‘grow’ as a function of plant biomass, plant species growth rates, and a plant-to-microbe conversion factor. Plant growth rates are a function of the proportion of different soil training types present. To prevent run-away growth, biomass is limited by a carrying capacity, which can be either unique to a species or to the community. Null model simulations are the same except that they include only one soil training type and one plant growth rate (Supplementary Note 1). The Null version of these models does not include a complementarity mechanism, but they can produce selection effects.
Growth rates were derived from (a) growth on control soils (control Null model), (b) growth on ‘self’ soils (self Null model), or (c) growth on each soil type (PSF model). Competition coefficients were assigned a value of ‘1’, but each species could affect the growth of other species due to community-level carrying capacities60. Each of these three model parameterizations (i.e. growth on control, growth on self, or growth on each soil type) was run with five different carrying capacities: (1) the maximum observed growth in any plot in the community experiment, (2) the maximum mean observed growth in any community, (3) the maximum species-specific growth in community plots, (4) the maximum observed growth in any PSF plot, and (5) the maximum species-specific growth in any PSF plot. Mean Null model predictions of community biomass were calculated from the 10 model simulations (Control Null, Self Null each with five carrying capacities). Mean PSF-model predictions were calculated from the five simulations with different carrying capacities.
Because growth rates were derived from the second year of growth, we assumed that growth rates represented 2 years of growth. To simulate the four years of growth in the biodiversity-productivity experiment, model simulations were executed for 52 timesteps, after which plant biomass was reduced to 1% of the previous timestep and allowed to run for another 52 timesteps. Model simulations for 52 or 208 timesteps produced qualitatively similar results but only results from the 104 timestep approach described immediately above are reported since they best represented conditions in the field. Mean model output for the sum of species growth from the suite of Null or PSF-model simulations are reported.
Parsing selection and complementarity effects
To better explain why biomass changes with species richness in each dataset (observed or predicted), the net change in community biomass with species richness was parsed into complementarity and selection effect components, using the modified Price equation (R package ‘partitionBEFsp”)11,33. Complementarity effects can be positive or negative, depending on whether species on average have higher or lower yields than the expected relative yield. Selection effects can be either positive or negative, depending on whether species have a positive or negative covariance between relative yield and biomass. This method is easily interpretable, comparable to other results, and remains the standard practice. Data from outlier communities with total biodiversity effects greater than five times the interquartile range were removed. Because S. rigida, D. purpurea, D. villosa, and D. candida did not grow in monoculture communities in BP1997, when partitioning biodiversity effects for BP1997, monoculture growth for these species was assumed to be twice biculture growth.
Testing PSF and biodiversity-productivity data
Patterns in the observed and predicted biomass with species richness were described with simple, best-fit log linear regressions (Proc Reg; SAS V9.4). The relationship between predicted and observed biomass in different plant communities was assessed by ordinary least squares regression. Plant-community biomass was the response variable that was predicted by either Null-or PSF-model-predicted biomass.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data used to prepare this manuscript can be found deposited at USU Digital Commons: https://doi.org/10.26078/52k0-jr94.
References
Tilman, D. et al. The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302 (1997).
Cardinale, B. J. et al. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl Acad. Sci. USA 104, 18123–18128 (2007).
Van Ruijven, J. & Berendse, F. Diversity-productivity relationships: Initial effects, long-term patterns, and underlying mechanisms. Proc. Natl Acad. Sci. USA 102, 695–700 (2005).
Jochum, M. et al. The results of biodiversity–ecosystem functioning experiments are realistic. Nat. Ecol. Evol. 4, 1485–1494 (2020).
Jing, J., Bezemer, T. M. & van der Putten, W. H. Complementarity and selection effects in early and mid-successional plant communities are differentially affected by plant-soil feedback. J. Ecol. 103, 641–647 (2015).
Tilman, D., Hill, J. & Lehman, C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 314, 1598–1600 (2006).
Mueller, K. E., Tilman, D., Fornara, D. A. & Hobbie, S. E. Root depth distribution and the diversity–productivity relationship in a long-term grassland experiment. Ecology 94, 787–793 (2013).
Hector, A., Bazeley-White, E., Loreau, M., Otway, S. & Schmid, B. Overyielding in grassland communities: testing the sampling effect hypothesis with replicated biodiversity experiments. Ecol. Lett. 5, 502–511 (2002).
Barry, K. E. et al. The future of complementarity: disentangling causes from consequences. Trends Ecol. Evol. 34, 167–180 (2019).
Kulmatiski, A., Beard, K. H. & Heavilin, J. Plant-soil feedbacks provide an additional explanation for diversity-productivity relationships. Proc. R. Soc. B Biol. Sci. 279, 3020–3026 (2012).
Loreau, M. & Hector, A. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76 (2001).
Tedersoo, L., Bahram, M. & Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 367, 6480 (2020).
Maron, J. L., Marler, M., Klironomos, J. N. & Cleveland, C. C. Soil fungal pathogens and the relationship between plant diversity and productivity. Ecol. Lett. 14, 36–41 (2011).
Wang, G. et al. Soil microbiome mediates positive plant diversity‐productivity relationships in late successional grassland species. Ecol. Lett. 22, 13273 (2019).
Wright, A. J., Wardle, D. A., Callaway, R. & Gaxiola, A. The overlooked role of facilitation in biodiversity experiments. Trends Ecol. Evol. 32, 383–390 (2017).
Bever, J. D., Platt, T. G. & Morton, E. R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 66, 265–283 (2012).
Bauer, J. T., Koziol, L. & Bever, J. D. Local adaptation of mycorrhizae communities changes plant community composition and increases aboveground productivity. Oecologia 192, 735–744 (2020).
Bever, J. D. Feeback between plants and their soil communities in an old field community. Ecology 75, 1965–1977 (1994).
Hendriks, M. et al. Independent variations of plant and soil mixtures reveal soil feedback effects on plant community overyielding. J. Ecol. 101, 287–297 (2013).
Zuppinger-Dingley, D. L., Flynn, D. F. B., De Deyn, G. B., Petermann, J. S. & Schmid, B. Plant selection and soil legacy enhance long-term biodiversity effects. Ecology 97, 15–0599.1 (2015).
Mommer, L. et al. Lost in diversity: the interactions between soil-borne fungi, biodiversity and plant productivity. N. Phytol. 218, 542–553 (2018).
Guerrero‐Ramírez, N. R., Reich, P. B., Wagg, C., Ciobanu, M. & Eisenhauer, N. Diversity‐dependent plant–soil feedbacks underlie long‐term plant diversity effects on primary productivity. Ecosphere 10, e02704 (2019).
van Ruijven, J., Ampt, E., Francioli, D. & Mommer, L. Do soil-borne fungal pathogens mediate plant diversity–productivity relationships? Evidence and future opportunities. J. Ecol. 108, 1810–1821 (2020).
Schnitzer, S. A. et al. Soil microbes drive the classic plant diversity–productivity pattern. Ecology 92, 296–303 (2011).
Lekberg, Y. et al. Relative importance of competition and plant-soil feedback, their synergy, context dependency and implications for coexistence. Ecol. Lett. 21, 1268–1281 (2018).
Cowles, J. Mechanisms of Coexistence: Implications for Biodiversity-Ecosystem Functioning Relationships in a Changing World. Dissertation, The University of Minnesota (2015).
Forero, L. E., Grenzer, J., Heinze, J., Schittko, C. & Kulmatiski, A. Greenhouse- and field-measured plant-soil feedbacks are not correlated. Front. Environ. Sci. 7, 184 (2019).
Kulmatiski, A. & Kardol, P. in Getting Plant—Soil Feedbacks out of the Greenhouse: Experimental and Conceptual Approaches 449–472 (Springer, 2008).
Pernilla Brinkman, E., Van der Putten, W. H., Bakker, E. J. & Verhoeven, K. J. F. Plant-soil feedback: experimental approaches, statistical analyses and ecological interpretations. J. Ecol. 98, 1063–1073 (2010).
van der Putten, W. H. et al. Plant-soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276 (2013).
Rinella, M. J. & Reinhart, K. O. Toward more robust plant-soil feedback research. Ecology 99, 550–556 (2018).
Crawford, K. M. et al. When and where plant‐soil feedback may promote plant coexistence: a meta‐analysis. Ecol. Lett. 22, 13278 (2019).
Clark, A. T. et al. How to estimate complementarity and selection effects from an incomplete sample of species. Methods Ecol. Evol. 10, 2141–2152 (2019).
Anacker, B. L., Klironomos, J. N., Maherali, H., Reinhart, K. O. & Strauss, S. Y. Phylogenetic conservatism in plant-soil feedback and its implications for plant abundance. Ecol. Lett. 17, 1613–1621 (2014).
Mehrabi, Z. & Tuck, S. L. Relatedness is a poor predictor of negative plant–soil feedbacks. N. Phytol. 205, 1071–1075 (2015).
Kulmatiski, A., Beard, K. H., Stevens, J. R. & Cobbold, S. M. Plant-soil feedbacks: a meta-analytical review. Ecol. Lett. 11, 980–992 (2008).
Beals, K. K. et al. Predicting plant-soil feedback in the field: meta-analysis reveals that competition and environmental stress differentially influence psf. Front. Ecol. Evol. 8, 191 (2020).
Kos, M., Tuijl, M. A. B., de Roo, J., Mulder, P. P. J. & Bezemer, T. M. Species-specific plant-soil feedback effects on above-ground plant-insect interactions. J. Ecol. 103, 904–914 (2015).
Bukowski, A. R. & Petermann, J. S. Intraspecific plant-soil feedback and intraspecific overyielding in Arabidopsis thaliana. Ecol. Evol. 4, 2533–2545 (2014).
Tilman, D., Wedin, D. & Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379, 718–720 (1996).
Fornara, D. A. & Tilman, D. Ecological mechanisms associated with the positive diversity–productivity relationship in an N-limited grassland. Ecology 90, 408–418 (2009).
Laughlin, D. C. et al. The hierarchy of predictability in ecological restoration: are vegetation structure and functional diversity more predictable than community composition? J. Appl. Ecol. 54, 1058–1069 (2017).
Metcalfe, H., Milne, A. E., Deledalle, F. & Storkey, J. Using functional traits to model annual plant community dynamics. Ecology 101, e03167 (2020).
Moulin, T., Perasso, A., Calanca, P. & Gillet, F. DynaGraM: a process-based model to simulate multi-species plant community dynamics in managed grasslands. Ecol. Modell. 439, 109345 (2021).
Putten, W. H., Bradford, M. A., Pernilla Brinkman, E., Voorde, T. F. J. & Veen, G. F. Where, when and how plant–soil feedback matters in a changing world. Funct. Ecol. 30, 1109–1121 (2016).
Eisenhauer, N., Reich, P. B. & Scheu, S. Increasing plant diversity effects on productivity with time due to delayed soil biota effects on plants. Basic Appl. Ecol. 13, 571–578 (2012).
Hawkes, C. V., Kivlin, S. N., Du, J. & Eviner, V. T. The temporal development and additivity of plant-soil feedback in perennial grasses. Plant Soil 369, 141–150 (2013).
Latz, E., Eisenhauer, N., Rall, B. C., Scheu, S. & Jousset, A. Unravelling linkages between plant community composition and the pathogen-suppressive potential of soils. Sci. Rep. 6, 1–10 (2016).
Chung, Y. A. & Rudgers, J. A. Plant–soil feedbacks promote negative frequency dependence in the coexistence of two aridland grasses. Proc. R. Soc. B Biol. Sci. 283 (2016).
Mahaut, L., Fort, F., Violle, C. & Freschet, G. T. Multiple facets of diversity effects on plant productivity: species richness, functional diversity, species identity and intraspecific competition. Funct. Ecol. 34, 287–298 (2020).
Barry, K. E. et al. Limited evidence for spatial resource partitioning across temperate grassland biodiversity experiments. Ecology 101, 2905 (2020).
Hooper, D. U. et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 (2005).
Pillai, P. & Gouhier, T. C. Not even wrong: the spurious measurement of biodiversity’s effects on ecosystem functioning. Ecology 100, e02645 (2019).
Manning, P. et al. Transferring biodiversity-ecosystem function research to the management of ‘real-world’ ecosystems. Adv. Ecol. Res. 61, 323–356 (2019).
Fargione, J. et al. From selection to complementarity: Shifts in the causes of biodiversity-productivity relationships in a long-term biodiversity experiment. Proc. R. Soc. B Biol. Sci. 274, 871–876 (2007).
Helander, M. et al. Decreases mycorrhizal colonization and affects plant-soil feedback. Sci. Total Environ. 642, 285–291 (2018).
Tilman, D. et al. Diversity and productivity in a long-term grassland experiment. Science 294, 843–845 (2001).
Cadotte, M. W., Cavender-Bares, J., Tilman, D. & Oakley, T. H. Using phylogenetic, functional and trait diversity to understand patterns of plant community productivity. PLoS ONE 4, e5695 (2009).
Kulmatiski, A., Heavilin, J. & Beard, K. H. Testing predictions of a three-species plant-soil feedback model. J. Ecol. 99, 542–550 (2011).
Kulmatiski, A., Beard, K. H., Grenzer, J., Forero, L. & Heavilin, J. Using plant-soil feedbacks to predict plant biomass in diverse communities. Ecology 97, 2064–2073 (2016).
Acknowledgements
This work was supported by grants from the US National Science Foundation DEB-1354129, and the Utah State University Ecology Center. This research was supported by the Utah Agricultural Experiment Station, Utah State University, and approved as journal paper number 9432. In addition, this work incorporated data from the Biodiversity II experiment at Cedar Creek Ecosystem Science Reserve, which is supported by grants from the US National Science Foundation Long-Term Ecological Research Program (LTER) including DEB-0620652 and DEB-1234162. Thanks to T. Mielke, K. Worm, J. Krueger, M. Saxhaug, J. Anderson, P. Barnes, L, Broome, J. Suvada, M. Berndt, A. Lindsey, J. Borchardt, B. Terry, J. Allenbrand, C. Pint, C. Carlisle, A. Brooks, A. Yamaguchi, A. Zlevor, L. Cherubini, P. Guevarra, L. Korte, M. Koenig, and C. Johnson for assistance with the field experiment. S. Durham and M. Holdrege provided statistical assistance. P. Adler, K. Beard, L. Kinkel, and S. Kuehl-Shelby provided manuscript feedback.
Author information
Authors and Affiliations
Contributions
L.F., A.K., J.G., and J.N. planned and designed the experiment. L.F., A.K., and J.G. performed the experiment. L.F. collected data. L.F. and A.K. collaborated in interpreting data. All authors contributed to the generation of ideas in this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Yuan Qin and Luke R. Grinham. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Forero, L.E., Kulmatiski, A., Grenzer, J. et al. Plant-soil feedbacks help explain biodiversity-productivity relationships. Commun Biol 4, 789 (2021). https://doi.org/10.1038/s42003-021-02329-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02329-1
This article is cited by
-
Dilution of specialist pathogens drives productivity benefits from diversity in plant mixtures
Nature Communications (2023)
-
A general stochastic model shows that plant-soil feedbacks can buffer plant species from extinction risks in unpredictable environments
Plant and Soil (2023)
-
Using root economics traits to predict biotic plant soil-feedbacks
Plant and Soil (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.