Abstract
Fine roots vary dramatically in their functions, which range from resource absorption to within-plant resource transport. These differences should alter resource availability to root-associated microorganisms, yet most root microbiome studies involve fine root homogenization. We hypothesized that microbial filtering would be greatest in the most distal roots. To test this, we sampled roots of six temperate tree species from a 23-year-old common garden planting, separating by branching order. Rhizoplane bacterial composition was characterized with 16S rRNA gene sequencing, while bacterial abundance was determined on a subset of trees through flow cytometry. Root order strongly impacted composition across tree species, with absorptive lower order roots exerting the greatest selective pressure. Microbial carrying capacity was higher in absorptive roots in two of three tested tree species. This study indicates lower order roots as the main point of microbial interaction with fine roots, suggesting that root homogenization could mask microbial recruitment signatures.
Similar content being viewed by others
Introduction
Plants shape the composition of root-adjacent (i.e., rhizosphere/rhizoplane) microorganisms through the release of root exudates and other rhizodeposits1,2,3,4,5, which creates a favorable environment for numerous microbial taxa2,5,6,7,8. Because of this environmental modification, clear differences in microbial composition are often observed between near-root environments and more distal “bulk” soil6,7,9,10,11. Differences between root-adjacent and bulk-soil microbial composition are likely driven by the selective pressure exerted in the root environment, as certain microbial taxa are preferentially recruited5,12. Further, the abundance of root exudates and rhizodeposits may facilitate a greater carrying capacity by providing nutrients to house a greater number of cells. Many root-adjacent microorganisms have been shown to influence plant growth and improve resilience to environmental perturbations13,14,15, but root–microbe relationships can also be commensal or deleterious in nature16,17,18. The vast majority of studies that investigate root-adjacent microbial composition have treated fine roots as equal during sampling6,7,11,19,20,21,22,23, which does not acknowledge the substantial structural and functional differences observed among different branching orders of the root system24. Homogenizing roots with differing functions may mask the microbial signals assigned to absorptive and transportive fine roots, which can skew interpretations of root-driven microbial filtering and recruitment.
Fine root classification has historically relied on size exclusion, in which roots below an arbitrarily chosen size (e.g., <2.0 mm diameter) were considered equivalent24,25,26. This approach can obscure between species comparisons, as it may combine roots with vastly different functions within a plant and different plant species possess disparate fine root morphologies24,25. An alternative approach is to classify fine roots according to branching order or functional role. Classification by root order involves designating the most distal roots as root order 1 (R1) and progressively increasing order numbers for root segments that grow closer to the base of the plant25,27. Functional classification involves separating fine roots according to their functional role, such as absorptive fine roots (typically includes R1/2) and transport fine roots (includes R4 and above)24,25. Structurally, absorptive and transportive roots differ with respect to root hair density, nutrient concentration, and morphology (e.g., development of cork periderm and senescence of root cortex with increasing root order)25,28,29. Functionally, lower root orders have greater absorptive capacity, respiration rate, and experience increased mycorrhizal colonization28,30,31, whereas higher root orders have greater transport capacity and life spans24,32,33.
Most trees form symbiotic relationships with either arbuscular mycorrhizal (AM) or ectomycorrhizal (EM) fungi34 and their colonization can vary greatly between absorptive and transportive fine roots30. Mycorrhizal fungi enhance the nutrient uptake capacity of their host35,36,37,38 and trees can specifically exploit these mycorrhizal symbioses to improve nutrient foraging39. Although mycorrhizal fungi are believed to interact with some soil bacteria40,41,42,43,44, their influence on bacterial composition is likely strongly attributed to leaf litter45. Specifically, the leaf litter from AM-associated trees decomposes significantly faster than their EM-associated counterparts46 and tree leaf litter can strongly influence the underlying soil properties45,47 and, subsequently, the bacterial composition. It is therefore likely that mycorrhizal associations can influence root-associated bacterial filtering and recruitment to some extent.
Redefining how fine roots influence the rhizosphere microbiome also has ecological implications. Differences in fine root function will create unique rhizosphere environments with differing selective pressures on microorganisms. For example, differences in decomposition48,49, root morphology25,29, water flux50, and nutrient content28,51 are evident among different root orders. Because of differences in fine root function, the composition of microorganisms associating with different root orders is likely to vary. Homogenizing different root orders, as is done in the vast majority of studies on the root microbiome, may obscure measures of plant investment in microbial selection and of fine-scale microbial filtering. Root homogenization could be particularly problematic when attempting to differentiate the influence of closely related plant genotypes on soil microbial recruitment. Studies have suggested that the phylogenetic signal of microbial recruitment can be relatively subtle11,23, but it may be that root homogenization is dulling or entirely obscuring such patterns.
We collected roots from six different temperate tree species that varied widely in mycorrhizal type and root diameter (three AM- and three EM-associating trees) following 23 years of growth in a common garden plantation, and separated these by root order. These tree species were chosen to maximize variations in root diameter and mycorrhizal association, which are both considered key root traits that influence root function52. Functionally distinct fine roots were then used to characterize the rhizoplane bacterial composition. We assessed the impact of root order and tree type on bacterial recruitment, as well as bacterial cell counts on a subset of the sampled trees. Our principal hypotheses were as follows: (i) bacterial taxa that have been previously linked to rapid carbon usage would be more prevalent in absorptive fine roots; (ii) absorptive fine roots, which have greater metabolic activity, would exert the greatest selective pressure (microbial filtering) on bacterial recruitment from bulk soil; and (iii) absorptive roots would have a greater microbial carrying capacity relative to transportive fine roots.
Results
Different root orders harbor unique microbial assemblages
To identify the influence of root order and mycorrhizal association on bacterial composition, we sampled six tree species (Fig. 1 and Supplementary Fig. 1) from a common garden planting. Root orders were determined according to the topological approach (e.g., see Pregitzer et al.25 and McCormack et al.24), and for each tree species, we separately collected R1/2 (absorptive), R3 (transitional), and R4/5 (transportive). In general, R1/2 represent newly developed roots with the greatest production of root exudates, R3 is a transitional root type, and R4/5 are typically thicker roots with a well-developed cork periderm that are involved in water and nutrient transport24,30.
Root orders are colored as follows: red is R1/2 (absorptive fine roots), green is R3 (transitionary fine roots), and blue is R4/5 (transportive fine roots). For an enhanced image of R1/2 for each tree species, please see Supplementary Fig. 1. Scale bar is 50 mm.
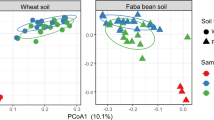
Bacterial composition, based on sequencing of the 16S rRNA gene region, differed significantly by root order (permutational multivariate analysis of variance (PERMANOVA); F2,103 = 1.4, R2 = 0.03, p = 0.04), which was principally driven by differences between R1/2 and R4/5 (F1,69 = 1.95, R2 = 0.03, p = 0.01; Supplementary Fig. 2). Significant heterogeneity was observed between different tree species within particular root orders (R1/2: F5,29 = 2.7, R2 = 0.32, p = 0.001; R3: F5,29 = 1.9, R2 = 0.24, p = 0.001; R4/5: F5,30 = 2.2, R2 = 0.26, p = 0.001), whereas differences between tree species appeared to decrease with increasing root order (Supplementary Fig. 3A, B). Differences between root orders were observed within all tree species (Fig. 2 and Table 1) and this was principally driven by differences between R1/2 and R3/R4/5 (Supplementary Table 1).
Soil bacterial compositions significantly differed overall (F5,30 = 1.5, R2 = 0.2, p = 0.001) but ordination plots indicated this difference was due to high variability in Carya glabra soil assemblages (Supplementary Fig. 3A, B). Removal of these samples also removed significance (F4,25 = 1.2, R2 = 0.16, p = 0.07). In all instances, soil microbial assemblages were distinct from their respective tree root orders, except for Liriodendron tulipifera where soil and R4/5 bacterial compositions were not different (Supplementary Table 2).
Microbial filtering of root bacterial composition decreases with increasing root order
We also aimed to determine whether absorptive roots, which are heavily involved in exchanging materials with microorganisms, were more dissimilar from bulk soil than the higher root orders, which would indicate stronger selection on the root-associated bacterial pool. To assess this, we extracted the Bray–Curtis dissimilarity distances of bulk soil samples to samples from each root order. To mitigate block effects, we only chose dissimilarity distances from within a given block (e.g., Block 1 soil vs. Block 1 Tree R1/2, R3, and R4/5; Fig. 3). Bacterial assemblages associated with R1/2 were the most distinct from bacterial assemblages in bulk soil (Bray dissimilarity median ± SE; 0.681 ± 0.006) followed by R3 (0.678 ± 0.006) and R4/5 (0.64 ± 0.007). We observed significant differences in the similarity of root-associated bacterial composition relative to bulk soil (Kruskal–Wallis test: H = 20, 2 d.f., p < 0.001), which was principally driven by R4/5 when compared to R1/2 (H = 15, 1 d.f., p < 0.001) and R3 (H = 15, 1 d.f., p < 0.001). For individual tree species (Supplementary Fig. 4), differences in root order similarity to soil were detected for Acer saccharum (H = 11, 2 d.f., p = 0.004), Juglans nigra (H = 11, 2 d.f., p = 0.004), L. tulipifera (H = 16, 2 d.f., p < 0.001), and C. glabra (H = 7, 2 d.f., p = 0.04), which was due to increased similarity of soil bacterial compositions and R4/5 relative to R1/2 (L. tulipifera and J. nigra) and R3 (A. saccharum, J. nigra, and C. glabra; Supplementary Table 3).
Root orders display stepwise increases or decreases in the relative abundance of specific bacterial phyla
To examine stepwise increases or decreases in taxa between root orders, we performed a similarity percentages (SIMPER) analysis at the phylum level between grouped root orders (Fig. 4). On average, members of the Proteobacteria, Bacteroidetes, Spirochaetes, and TM6 were found to progressively decrease with increasing root order, with the Proteobacteria decrease primarily driven by the Betaproteobacteria (Supplementary Table 4 and Supplementary Fig. 5). In contrast, members of the Acidobacteria, Verrucomicrobia, Plantomycetes, Gemmatimonadetes, Elusimicrobia, OD1, and Firmicutes were found to increase with increasing root order. In addition, we sought to examine conserved sequences across all root samples. We identified four operational taxonomic units (OTUs) as being present in every root sample. These four OTUs were assigned as members of the Rhodoplanes (OTU 189524077), DA101 (of the Verrucomicrobia phylum; OTU 229398176), Bradyrhizobium (OTU 374925622), and Mycobacterium genera (OTU 814675324), representing an average relative abundance of 0.7%, 2.3%, 3.9%, and 0.6%, respectively.
Microbial assemblages differ according to mycorrhizal association
When separated according to mycorrhizal association (AM vs. EM), bacterial compositions were distinct when grouping all root orders together (F1,104 = 8.5, R2 = 0.08, p = 0.001) and when examining root orders separately (R1/2: F1,33 = 4.2, R2 = 0.11, p = 0.001; R3: F1,33 = 4.2, R2 = 0.08, p = 0.002; R4/5: F1,34 = 3.4, R2 = 0.09, p = 0.001). Bacterial composition differences between AM- and EM-associating trees were driven by a consistent over-representation of the Acidobacteria in each EM-associating tree root order (R1/2: 16% dissimilarity contribution; R3: 18% dissimilarity contribution; and R4/5: 15% dissimilarity contribution). These patterns confirm our recent observations for absorptive lower-order roots between AM- and EM-associating trees53, but also show that these patterns hold true for transitionary and transportive fine roots.
Absorptive roots house greater numbers of bacteria
As absorptive fine roots have more nutrient and water influx relative to transportive fine roots, we expect that the microbial carrying capacity could be higher in the absorptive fine roots. Therefore, to quantify bacterial cell counts between root orders, we performed flow cytometry on three tree species. The tree species J. nigra, L. tulipifera, and Pinus strobus were chosen, as they harbored the most distinct bacterial compositions at their absorptive roots (Supplementary Fig. 3A, B). Overall, bacterial cell counts were significantly higher in J. nigra (mean ± SE per gram of dry root; 2.6 × 107 ± 4.5 × 106) relative to both L. tulipifera (7.4 × 106 ± 2.3 × 106; Kruskal–Wallis test: H = 12.6, 1 d.f., p < 0.001) and P. strobus (9.0 × 106 ± 3.8 × 106; H = 10.6, 1 d.f., p = 0.001). When comparing grouped root orders, R1/2 (2.4 × 107 ± 5.7 × 106) had the highest bacterial cell counts when compared to R4/5 (8.6 × 106 ± 2.5 × 106; H = 4.6, 1 d.f., p = 0.03) with R3 intermediate (9.7 × 106 ± 2.6 × 105). Lack of significance between R1/2 and R3 across all three tree species was primarily driven by highly variable cell counts recorded for L. tulipifera. Because of this variability, no differences were observed between individual root orders for L. tulipifera (Fig. 5). However, both J. nigra and P. strobus had significantly elevated cell counts in R1/2 relative to both R3 (H = 5.8, 1 d.f., p = 0.02; H = 3.9, 1 d.f., p = 0.05; respectively) and R4/5 (H = 5.8, 1 d.f., p = 0.02; H = 5.8, 1 d.f., p = 0.009; respectively).
Discussion
The way in which we define and sample fine roots has broad implications for our understanding of plant-, ecosystem-, and global-scale processes, including nutrient and carbon cycling24. Nearly all microbiome-focused studies of root systems involve homogenization of multiple root orders6,7,11,19,20,21,22,23, likely because of the laborious nature of sorting fine roots. In root biology studies that do attempt to distinguish between root types, traditional approaches to root classification have relied primarily on size exclusion, wherein all roots below a particular size (e.g., 2.0 mm diameter) were considered equivalent25. However, such size-exclusion approaches do not account for substantial cross-species variability in root morphology or the considerable variability in functional roles within the branches of a fine root system24,25. For example, a 2.0 mm diameter classification would include all fine roots up to the fourth root order for L. tulipifera24.
Our data demonstrate clear differences in bacterial composition according to root order for six phylogenetically diverse tree species, which vary widely in fine root traits (Fig. 6). We expect that these differences are likely driven by differences in the functional role of each root order. In newly developed absorptive roots, we expect higher respiration and increased flux of labile carbon, water, and nutrients relative to the longer lived but less absorptive transportive fine roots24,32. Supporting this, we observed a greater relative abundance of higher taxa associated with efficient carbon mineralization in absorptive roots, namely the Betaproteobacteria and Bacteroidetes54, and an under-representation of the Acidobacteria, which have been shown to correlate negatively with carbon mineralization54. Recent developments for our understanding of fine root dynamics in soil have identified differences in fine root morphology and turnover rates according to soil depth, and seasonal effects on fine root production, biomass, necromass, and mortality55. Further, nonlinear relationships between root diameter, tissue density, and nitrogen concentration have been recently identified for woody plant species56. As our understanding of fine root dynamics improves so too will our understanding of the conditions required for microbial colonization and proliferation. For example, and depending on the season of sampling, higher rates of fine root mortality could shift microbial dynamics towards decomposers, which could alter compositional differences between branching root orders.
The composition of root-associated bacteria was most distinct from bulk soil in the lowest root orders, where bacterial abundance was also typically higher. Taxa associated with carbon mineralization were found in greater relative abundance in root orders 1 and 2, suggesting that copiotrophic phyla preferentially colonize absorptive fine roots with greater nutrient flux. R1/2 is colored orange, R3 is green, and R4/5 is blue.
As absorptive roots are a hotspot for nutrient flux31,57, we expected a greater microbial carrying capacity in these microenvironments. We used flow cytometry to quantify bacterial cells from three of the six sampled trees, and results from two of these provided a strong support for our expectation. For J. nigra and P. strobus, R1/2 harbored the greatest bacterial abundance relative to R3 and R4/5. The bacterial abundance was almost an order of magnitude greater for P. strobus and nearly three times greater for J. nigra when compared to R4/5. This suggests that absorptive fine roots, at least in some species, have a greater microbial carrying capacity relative to transitionary and transportive fine roots. The increased microbial carrying capacity is likely directly related to the greater nutrient flux in absorptive fine roots31,57 and corresponds to increases in taxa known for their copiotrophic lifestyles (e.g., Betaproteobacteria and Bacteroidetes)54. These data further highlight the need for differentiating fine roots according to their functional roles, as root homogenization can combine fine roots with extremely disproportionate bacterial abundance (e.g., fine root bacterial abundance as almost an order of magnitude different for P. strobus).
With increasing root order, root-associated microbial composition converged on those observed in bulk soil. This suggests that transportive fine roots exert less selective pressure on microorganisms than absorptive fine roots, which is also supported by our observation of increased cell abundance in the absorptive fine roots of two of three tree tested tree species. Selective bacterial recruitment by absorptive roots could be driven by root exudates and rhizodeposits4, which are known to influence microbial community composition3,4,22. Methods for root sampling can therefore significantly impact the composition of observed microorganisms and our data suggest that homogenization or size exclusion could dull patterns of microbial selection. This has important impacts on our understanding of plant-driven microbial recruitment and, in particular, our ability to detect differences in microbial selection across closely related plant genotypes. Although our data demonstrate that homogenization of different root orders can dull microbial signals and lead to erroneous interpretations of microbial data, we did not include a “homogenization” control as a comparative sample. The magnitude of error from homogenization is difficult to predict and will ultimately be strongly influenced by the surface area contribution by each root order in the homogenized sample. In a typical four to five branching root system, absorptive fine roots contribute a greater proportion to root length and quantity (number of segments), whereas transportive fine roots contribute a greater proportion to root mass25,58. The proportionally higher abundances of absorptive fine roots may diminish errors when homogenizing roots, but this would be species specific. Regardless, we observed differences in microbial patterns across root orders and these microbial signals would be diluted by combining multiple root orders into one sample.
In addition to those bacterial composition differences observed between different fine roots, we also identified broad patterns separating microbial assemblages for AM- and EM-associating trees for each individual root order. In support of our data, differences between bacterial compositions for AM- and EM-associating trees for absorptive fine roots have been recently noted53 and our data highlight that this pattern is consistent for higher-order roots. Close associations between mycorrhizal fungi and particular bacterial taxa have been previously noted40,41,42,43,44,59,60, which could explain the bacterial composition separation observed in this study. We found that members of the Acidobacteria were consistently over-represented in EM-associating plants relative to AM-associating plants for each root order. In agreement with this finding, Acidobacteria are notable for their ability to suppress AM fungi activity41.
Although we observed clear differences in bacterial composition, microbial filtering, and microbial carrying capacity according to branching root order, caution should be applied when generalizing these patterns to natural conditions. Our experimental design used a 23-year-old common garden planting, and while soil conditions were homogenous at the time of planting61, these conditions may not necessarily reflect the response of plants growing in natural conditions. Certainly, our common garden planting would be considered a partly controlled environment and differences in root traits, such as fine‐root tissue density, have been identified previously when compared to natural settings62. However, root trait similarities between common garden plantings and natural conditions have been observed for nitrogen concentration, root diameter, and root length62.
Differing approaches to fine root sampling can dramatically alter our view of belowground processes. In recent years, assessments of root-associated microbiomes have dramatically increased in number and most of these rely on homogenization of root clusters in our systems. This study identified significant differences in bacterial composition between fine root orders, with often significantly elevated microbial cell counts in absorptive fine roots relative to transportive fine roots. These data highlight differences in microbial carrying capacity, and root-driven microbial filtering and selective pressure of bacterial composition. These differences are notable for experimental designs which homogenize fine roots with different functionality, as this can mask the observable microbial patterns, particularly if the microbial signal is subtle. Accurate classification of fine roots according to their functional roles has ecological implications for a number of different fields, particularly microbiology. Here we have shown differences in microbial carrying capacity, microbial filtering and selective pressure, and bacterial compositions for functionally discrete fine roots. Therefore, future studies aiming to characterize plant–microbe–environment interactions will need to account for varying fine root orders, to ensure subtle microbial signals are not masked by homogenization and to avoid spurious interpretations of their microbial patterns.
Methods
Study site
The experimental site was a common garden forest located at the Russell E. Larson Agricultural Research Center in central Pennsylvania (40°42′ N, 77°57′ W), which has previously been described in detail61. Briefly, this common garden forest contains 16 different tree species, which were planted as 1-year-old seedlings in 1996 and was constructed with a randomized complete block design. Soil conditions were homogenous throughout the site prior to seedling planting61. For this study, we selected three AM-associating (A. saccharum, J. nigra, and L. tulipifera) and EM-associating (C. glabra, Quercus rubra, and P. strobus) tree species that represented a variety of taxonomic groups and root traits.
Sample collection
Roots and bulk soil were sampled in 2018 on 3 July (blocks 1–4) and 13 July (blocks 5 and 6). Sampling was performed on two separate days because of the labor-intensive nature of sampling. Roots were collected using a spading fork and gently shaken to remove loosely adherent soil. Root order was determined by the topological approach (e.g., see Pregitzer et al.25 and McCormack et al.24). For each tree species, we collected R1/2 (absorptive), R3 (transitional), and R4/5 (transportive) from two root clusters for each single-species plot from each of the six blocks. Samples were transported to the laboratory on ice and stored at −20 °C until needed.
Soil organic matter was uniform across the common garden plantation, with differences in pH observed for some tree species53. Absorptive root diameter did not explain differences in soil properties between species53. Measures of root branching ratio, branching intensity, and root diameter are provided in Supplementary Table 5.
DNA extraction and sequencing
Sampled roots were transferred into a 1.5 ml NucleoSpin® bead tube and 700 µL of lysis buffer (SL1) was added. Samples were sonicated for 5 min (Branson Bransonic Ultrasonic Bath) and the roots were subsequently removed. DNA was extracted from bulk soil samples and the sonicated solutions using a NucleoSpin® 96 soil DNA extraction kit (catalog: 740787.2) according to the manufacturer’s instructions.
Extracted DNA were amplified using the 515F63 and 806R64 primer pair, as previously described65. Briefly, PCR ingredients were as follows: 8 μl of 5Prime HotMasterMix (Quanta BioSciences, Inc.), 1 μl template DNA, 1 μl of each primer (10 μM), and 9 μl molecular grade water, for a final volume of 20 μl. PCR cycling conditions were as follows: 94 °C for 3 min, 25 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s, and a final elongation step for 10 min at 72 °C. Amplicons were then purified with Mag-Bind TotalPure NGS clean-up beads (Omega Bio-Tek). A second PCR was used to add Illumina adapters and indexing barcodes to the purified amplicons. The second PCR ingredients were as follows: 5 μl of cleaned PCR product, 12.5 μl of 5Prime HotMasterMix, 2.5 μl of water, and 2.5 μl of each index primer (10 μM), for a final volume of 25 μl. The second PCR conditions were as follows: 98 °C for 1 min, 8 cycles of 98 °C for 15 s, 55 °C for 30 s, and 72 °C for 20 s, followed by a final elongation step for 5 min at 72 °C.
Barcoded and indexed amplicons were normalized using a SequalPrep Normalization Plate Kit (Invitrogen) and pooled. Pooled amplicons were concentrated using a Savant SpeedVac (Thermo Scientific) at 50 °C for 3 h. Concentrated DNA was run in a 1.2% agarose gel and the expected band was excised and extracted using a PureLink Quick Gel Extraction Kit (Invitrogen). Purified pooled amplicons were sent to the Cornell University Biotechnology Resource Center Genomics Facility to be sequenced on the Illumina MiSeq platform (2 × 250 cycle v2 kit). Raw data files in FASTQ format were deposited in the NCBI sequence read archive under Bioproject number PRJNA639455. In total, we characterized the bacterial composition of 108 root samples and 36 bulk soil samples targeting the 16S rRNA V4 region.
Sequencing analysis
Raw fastq data were processed with Mothur66 (version 1.36) and QIIME67 (version 1.9.1). In Mothur, paired end reads were merged with make.contigs. trimmed with trim.seqs (pdiffs = 2) and singletons removed using split.abund. In QIIME, joined reads were dereplicated, clustered at the 97% threshold, and chimeras removed using USEARCH68. Taxonomy was assigned in Mothur against the GreenGenes69 (version 13.8.99) database. Processed data were then imported into the R statistical environment and further cleaned. First, OTUs assigned as mitochondria, archaea, chloroplasts, and unclassified phyla were removed. Data were then rarefied to 5427 counts per sample, which was chosen to preclude the inclusion of two samples with low counts (38 and 191 counts). Data were then transformed compositionally (relative abundance) and used to produce a Phyloseq70 object for further analyses.
Flow cytometry
To quantify microbial cell counts between tree species and root orders, we quantified cells in the rhizoplane and rhizoplane soil using flow cytometry. We chose three tree species J. nigra, L. tulipifera, and P. strobus, as they harbored the most distinct bacterial compositions at their absorptive roots (Supplementary Fig. 3A, B). Fresh root samples were collected in the following year and were stored at 4 °C prior to cell extraction from the rhizoplane and rhizoplane soil. Equal mass of root samples for each root order were visually assessed and transferred into a 1.5 ml tube. The cell extraction was performed as previously described71. Briefly, 700 μl of sterile NaCl 0.85% solution was added into each tube and the roots were sonicated (Branson Bransonic Ultrasonic Bath) for 5 min. Sonication removed the rhizoplane microbes from the root surface into the NaCl solution. Roots were then removed from the sample and each sample was homogenized by vortexing for 5 min at full speed. The homogenized suspension was centrifuged at 130 × g for 5 min, to exclude the large soil particles. The supernatant was then filtered at 40 μm, to remove particles for the further analyses. From this filtered solution, we took a 250 μl aliquot and stained it with 1 μL of SYBR® Green I (10,000× in dimethyl sulfoxide; Life Technologies). All samples were incubated at room temperature for 15 min in the dark. Absolute counts were achieved by using a Flow-Count Fluorosphere according to the manufacturer’s instructions. Cells were quantified using a MACSQuant Vyb flow cytometer (Miltenyi) with a 488 nm blue laser and a 525/50 nm channel.
Statistics and reproducibility
To compare bacterial assemblages between root orders and differing fungal mycorrhizal associated trees, a principal coordinates analysis (PCoA) with a Bray–Curtis dissimilarity index was used. To examine root orders within tree species, a constrained canonical analysis of principal coordinates (CAP) was used with a Bray–Curtis dissimilarity index. PCoA and CAP were performed using the ordinate function in the Phyloseq package70. Patterns elucidated by the PCoA were statistically tested using Adonis (PERMANOVA) from the vegan package72 with 999 permutations. To identify taxa driving the difference between groups, a SIMPER analysis was used from the vegan package with a Bray–Curtis dissimilarity index. Data were summarized at different taxonomic levels using the MicrobeR package73. Comparisons of flow cytometry cell count data were performed using a Kruskal–Wallis test from the stats package74. To compare Bray–Curtis dissimilarities of root orders relative to soil, a Kruskal–Wallis test from the stats package was used, followed by a Dunnett’s post hoc test. All statistical tests were performed in the R statistical environment74. Sample sizes were as follows: six blocks, six tree species (three AM- and three EM-associating) per block, and three root orders or bulk soil from each tree species (six replicates for each root order or bulk soil per tree species). After rarefaction, two replicates were removed (L. tulipifera R1/2 and J. nigra R3). For flow cytometry cell counts, each root order for each tree species had five replicates.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Raw data files in FASTQ format were deposited in the NCBI sequence read archive under Bioproject number PRJNA639455 and supporting data for this manuscript can be found in Supplementary Data 1.
References
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A. & Dufresne, A. The importance of the microbiome of the plant holobiont. N. Phytol. 206, 1196–1206 (2015).
Feng, H. et al. Identification of chemotaxis compounds in root exudates and their sensing chemoreceptors in plant-growth-promoting Rhizobacteria Bacillus amyloliquefaciens SQR9. Mol. Plant Microbe Interact. 31, 995–1005 (2018).
Dennis, P. G., Miller, A. J. & Hirsch, P. R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 72, 313–327 (2010).
Walker, T. S., Bais, H. P., Grotewold, E. & Vivanco, J. M. Root exudation and rhizosphere biology. Plant Physiol. 132, 44 (2003).
Zhalnina, K. et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 3, 470–480 (2018).
Bulgarelli, D. et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488, 91–95 (2012).
Schreiter, S. et al. Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce. Front. Microbiol. 5, 144 (2014).
Zhang, N. et al. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 374, 689–700 (2014).
Yang, C.-H. & Crowley, D. E. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl. Environ. Microbiol. 66, 345 (2000).
DeAngelis, K. M. et al. Selective progressive response of soil microbial community to wild oat roots. ISME J. 3, 168–178 (2009).
Peiffer, J. A. et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl Acad. Sci. USA 110, 6548 (2013).
Shi, S. et al. Successional trajectories of rhizosphere bacterial communities over consecutive seasons. mBio 6, e00746–00715 (2015).
Lu, T. et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 6, 231 (2018).
Mei, C. & Flinn, B. S. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat. Biotechnol. 4, 81–95 (2010).
Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 26, 209–214 (2016).
Waschkies, C., Schropp, A. & Marschner, H. Relations between grapevine replant disease and root colonization of grapevine (Vitis sp.) by fluorescent pseudomonads and endomycorrhizal fungi. Plant Soil 162, 219–227 (1994).
Benizri, E. et al. Replant diseases: bacterial community structure and diversity in peach rhizosphere as determined by metabolic and genetic fingerprinting. Soil Biol. Biochem. 37, 1738–1746 (2005).
Pankhurst, C. E. et al. Management practices to improve soil health and reduce the effects of detrimental soil biota associated with yield decline of sugarcane in Queensland, Australia. Soil Tillage Res. 72, 125–137 (2003).
Fitzpatrick, C. R. et al. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc. Natl Acad. Sci. USA 115, E1157 (2018).
Zhang, Y. et al. Huanglongbing impairs the rhizosphere-to-rhizoplane enrichment process of the citrus root-associated microbiome. Microbiome 5, 97 (2017).
Edwards, J. et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl Acad. Sci. USA 112, E911 (2015).
Hu, L. et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 9, 2738 (2018).
Lundberg, D. S. et al. Defining the core Arabidopsis thaliana root microbiome. Nature 488, 86–90 (2012).
McCormack, M. L. et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. N. Phytol. 207, 505–518 (2015).
Pregitzer, K. S. et al. Fine root architecture of nine North American trees. Ecol. Monogr. 72, 293–309 (2002).
Holdaway, R. J., Richardson, S. J., Dickie, I. A., Peltzer, D. A. & Coomes, D. A. Species- and community-level patterns in fine root traits along a 120 000-year soil chronosequence in temperate rain forest. J. Ecol. 99, 954–963 (2011).
Fitter, A. H. Morphometric analysis of root systems: application of the technique and influence of soil fertility on root system development in two herbaceous species. Plant Cell Environ. 5, 313–322 (1982).
Valenzuela-Estrada, L. R., Vera-Caraballo, V., Ruth, L. E. & Eissenstat, D. M. Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae). Am. J. Bot. 95, 1506–1514 (2008).
Hishi, T. Heterogeneity of individual roots within the fine root architecture: causal links between physiological and ecosystem functions. J. For. Res. 12, 126–133 (2007).
Guo, D. et al. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. N. Phytol. 180, 673–683 (2008).
Makita, N. et al. Fine root morphological traits determine variation in root respiration of Quercus serrata. Tree Physiol. 29, 579–585 (2009).
Guo, D., Mitchell, R. J., Withington, J. M., Fan, P.-P. & Hendricks, J. J. Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: root branch order predominates. J. Ecol. 96, 737–745 (2008).
Gu, J., Yu, S., Sun, Y., Wang, Z. & Guo, D. Influence of root structure on root survivorship: an analysis of 18 tree species using a minirhizotron method. Ecol. Res. 26, 755–762 (2011).
Wang, B. & Qiu, Y. L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16, 299–363 (2006).
Tibbett, M. & Sanders, F. E. Ectomycorrhizal symbiosis can enhance plant nutrition through improved access to discrete organic nutrient patches of high resource quality. Ann. Bot. 89, 783–789 (2002).
Sanders, F. E. & Tinker, P. B. Phosphate flow into mycorrhizal roots. Pestic. Sci. 4, 385–395 (1973).
Hodge, A. & Storer, K. Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386, 1–19 (2015).
Bending, G. D. & Read, D. J. The structure and function of the vegetative mycelium of ectomycorrhizal plants. N. Phytol. 130, 401–409 (1995).
Chen, W. et al. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl Acad. Sci. USA 113, 8741 (2016).
Gui, H., Hyde, K., Xu, J. & Mortimer, P. Arbuscular mycorrhiza enhance the rate of litter decomposition while inhibiting soil microbial community development. Sci. Rep. 7, 42184–42184 (2017).
Svenningsen, N. B. et al. Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME J. 12, 1296–1307 (2018).
Olsson, P. A. & Wallander, H. Interactions between ectomycorrhizal fungi and the bacterial community in soils amended with various primary minerals. FEMS Microbiol. Ecol. 27, 195–205 (1998).
Hestrin, R., Hammer, E. C., Mueller, C. W. & Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2, 233 (2019).
Garbaye, J. Helper bacteria: a new dimension to the mycorrhizal symbiosis. N. Phytol. 128, 197–210 (1994).
Phillips, R. P., Brzostek, E. & Midgley, M. G. The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. N. Phytol. 199, 41–51 (2013).
Cornelissen, J., Aerts, R., Cerabolini, B., Werger, M. & van der Heijden, M. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia 129, 611–619 (2001).
Reich, P. B. et al. Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecol. Lett. 8, 811–818 (2005).
Minerovic, A. J., Valverde-Barrantes, O. J. & Blackwood, C. B. Physical and microbial mechanisms of decomposition vary in importance among root orders and tree species with differing chemical and morphological traits. Soil Biol. Biochem. 124, 142–149 (2018).
Fan, P. & Guo, D. Slow decomposition of lower order roots: a key mechanism of root carbon and nutrient retention in the soil. Oecologia 163, 509–515 (2010).
Segal, E., Kushnir, T., Mualem, Y. & Shani, U. Water uptake and hydraulics of the root hair rhizosphere. Vadose Zone J. 7, 1027–1034 (2008).
Gordon, W. S. & Jackson, R. B. Nutrient concentrations in fine roots. Ecology 81, 275–280 (2000).
Ma, Z. et al. Evolutionary history resolves global organization of root functional traits. Nature 555, 94–97 (2018).
Yates, C. F. et al. Tree‐induced alterations to soil properties and rhizoplane‐associated bacteria following 23 years in a common garden. Plant Soil, https://doi.org/10.1007/s11104-021-04846-8 (2021).
Fierer, N., Bradford, M. A. & Jackson, R. B. Toward an ecological classification of soil bacteria. Ecology 88, 1354–1364 (2007).
Wang, N., Wang, C. & Quan, X. Variations in fine root dynamics and turnover rates in five forest types in northeastern China. J. Forestry Res. 31, 871–884 (2020).
Kong, D. et al. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 10, 2203 (2019).
Jia, S., Wang, Z., Li, X., Zhang, X. & McLaughlin, N. B. Effect of nitrogen fertilizer, root branch order and temperature on respiration and tissue N concentration of fine roots in Larix gmelinii and Fraxinus mandshurica. Tree Physiol. 31, 718–726 (2011).
Lavely, E. K. et al. On characterizing root function in perennial horticultural crops. Am. J. Botany, https://doi.org/10.1002/ajb2.1530 (2020).
Iffis, B., St-Arnaud, M. & Hijri, M. Bacteria associated with arbuscular mycorrhizal fungi within roots of plants growing in a soil highly contaminated with aliphatic and aromatic petroleum hydrocarbons. FEMS Microbiol. Lett. 358, 44–54 (2014).
Toljander, J. F., Lindahl, B. D., Paul, L. R., Elfstrand, M. & Finlay, R. D. Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiol. Ecol. 61, 295–304 (2007).
McCormack, M., Adams, T. S., Smithwick, E. A. H. & Eissenstat, D. M. Predicting fine root lifespan from plant functional traits in temperate trees. N. Phytol. 195, 823–831 (2012).
Freschet, G. T. et al. Climate, soil and plant functional types as drivers of global fine-root trait variation. J. Ecol. 105, 1182–1196 (2017).
Parada, A. E., Needham, D. M. & Fuhrman, J. A. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 18, 1403–1414 (2016).
Apprill, A., McNally, S., Parsons, R. J. & Weber, L. K. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 75, 129–137 (2015).
Trexler, R. V. & Bell, T. H. Testing sustained soil-to-soil contact as an approach for limiting the abiotic influence of source soils during experimental microbiome transfer. FEMS Microbiol. Lett. 366, https://doi.org/10.1093/femsle/fnz228 (2019).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537 (2009).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
DeSantis, T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069 (2006).
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLOS ONE 8, e61217 (2013).
Bressan, M. et al. A rapid flow cytometry method to assess bacterial abundance in agricultural soil. Appl. Soil Ecol. 88, 60–68 (2015).
Oksanen, J. et al. Vegan: community ecology package. R. Package Version 2. 2-1 2, 1–2 (2015).
Bisanz, J. E. MicrobeR: Handy functions for microbiome analysis in R. (2019).
R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2012).
Acknowledgements
This research was supported by the USDA National Institute of Food and Agriculture (NIFA) Foundational Program (Accession #1014758) and by the USDA NIFA Federal Appropriation under Project #PEN0 4628 (Accession #1014131), Project #PEN0 4744 (Accession #1023222), and Project #PEN0 4651 (Accession #1016233). Partial support was also provided by the China Scholarship Council. We thank Timothy Peoples, Franco Acevedo Luco, Jeremy Harper, Amanda Seow, and Wenqi Luo for their help with root sampling, and Kevin Hockett for the use of the sonic water bath and technical advice.
Author information
Authors and Affiliations
Contributions
J.G., S.F., T.H.B., M.C., and D.M.E. collected the samples. J.G., C.Y., and S.F. processed the samples. R.V.T., J.G., and C.Y. produced the sequencing data. W.L.K. and M.C. analyzed the data. J.G. performed the flow cytometry. W.L.K., C.Y., and T.H.B. wrote the manuscript. M.C., T.H.B., and D.M.E. designed the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
King, W.L., Yates, C.F., Guo, J. et al. The hierarchy of root branching order determines bacterial composition, microbial carrying capacity and microbial filtering. Commun Biol 4, 483 (2021). https://doi.org/10.1038/s42003-021-01988-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-01988-4
This article is cited by
-
Endophytic microbiota and ectomycorrhizal structure of Alnus glutinosa Gaertn. at saline and nonsaline forest sites
Scientific Reports (2023)
-
Decomposing the novel decomposer-sphere concept: decomposition byproducts can shape surrounding communities through space and time
Biogeochemistry (2023)
-
Biotic Interactions in Soil are Underestimated Drivers of Microbial Carbon Use Efficiency
Current Microbiology (2023)
-
Functionally-explicit sampling can answer key questions about the specificity of plant–microbe interactions
Environmental Microbiome (2022)
-
Soil texture is a stronger driver of the maize rhizosphere microbiome and extracellular enzyme activities than soil depth or the presence of root hairs
Plant and Soil (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.