Abstract
The resurrection of pseudogenes during evolution produced lncRNAs with new biological function. Here we show that pseudogene-evolution created an Oct4 pseudogene lncRNA that is able to direct epigenetic silencing of the parental Oct4 gene via a 2-step, lncRNA dependent mechanism. The murine Oct4 pseudogene 4 (mOct4P4) lncRNA recruits the RNA binding protein FUS to allow the binding of the SUV39H1 HMTase to a defined mOct4P4 lncRNA sequence element. The mOct4P4-FUS-SUV39H1 silencing complex holds target site specificity for the parental Oct4 promoter and interference with individual components results in loss of Oct4 silencing. SUV39H1 and FUS do not bind parental Oct4 mRNA, confirming the acquisition of a new biological function by the mOct4P4 lncRNA. Importantly, all features of mOct4P4 function are recapitulated by the human hOCT4P3 pseudogene lncRNA, indicating evolutionary conservation. Our data highlight the biological relevance of rapidly evolving lncRNAs that infiltrate into central epigenetic regulatory circuits in vertebrate cells.
Similar content being viewed by others
Introduction
Pseudogenes are non-functional gene copies that have lost protein coding potential. Precise annotation and integration of functional genomics data revealed a high number of pseudogenes that have evolved to new functional elements, producing long noncoding RNAs (lncRNAs) in a tightly controlled manner1,2. In many cases, sequence similarity of pseudogene derived lncRNAs with parental gene transcripts provides the rational basis for pseudogene dependent control of ancestral gene expression. Pseudogene lncRNAs have been reported to compete with parental gene transcripts for miRNAs or RNA binding proteins or, alternatively, can give rise to endo-siRNAs3,4,5,6,7,8. Antisense transcription of pseudogenes can mediate epigenetic silencing of ancestral genes in trans, presumably by pairing with ancestral sense gene transcripts9,10. Remarkably, pseudogene derived lncRNAs have also been demonstrated to act as scaffold for chromatin modifying complexes that can modulate gene expression at multiple loci across the genome11,12.
The transcription factor OCT4 is central for vertebrate embryonic stem cell (ESC) pluripotency and cancer cell biology and represents a hallmark model for the multifaceted pathways of pseudogene lncRNA mediated regulation of parental gene expression10,13,14,15,16,17,18,19,20,21,22,23. During evolution, the murine and human Pou5f1/POU5F1 genes, that encode OCT4, gave rise to five processed murine (Pou5F1P1–Pou5F1P5) and eight processed human pseudogenes (POU5F1P1–POU5F1P8), with validated lncRNA expression17,24,25,26. Hereinafter, Pou5f1 and POU5F1 pseudogenes will be referred to as Oct4/OCT4 pseudogenes. Murine Oct4 pseudogene derived lncRNAs show defined pattern of expression during mouse embryonic stem cells (mESC) differentiation and specific cytoplasmic or nuclear localization, supporting evidence for the acquisition of new biological function17. In line with this, human OCT4 pseudogene 4 and 5 lncRNAs alter ancestral gene expression by acting as classic ceRNAs, and pairing of the murine Oct4-pseudogene 5 antisense lncRNA with Oct4 transcripts has a role in guiding the histone methyltransferase (HMTase) EZH2 to the OCT4 promoter10,16,27.
We recently reported on a new mechanism of ancestral gene regulation that depends on pseudogene lncRNA dependent recruitment of an epigenetic silencing complex to the Oct4 promoter in trans17. Induction of mESC differentiation results in efficient upregulation of the X-linked mOct4P4 gene that encodes the mOct4P4 lncRNA. The resulting nuclear restricted mOct4P4 lncRNA forms a complex with the HMTase SUV39h1 and targets H3K9me3 and HP1 to the promoter of the parental Oct4 gene on chromosome 17, leading to gene silencing in trans. Importantly, this mechanism does not involve pairing of Oct4 sense and pseudogene antisense RNAs. To this end, lncRNA sequence determinants and evolutional importance for mOct4P4 pseudogene lncRNA dependent silencing of Oct4 are not known.
Here, we show that the human POU5F1P3 pseudogene derived lncRNA, hOCT4P3, is a functional homolog of the murine Pou5f1P4 lncRNA in OVCAR-3 ovarian cancer cells, demonstrating evolutionally constraint on pseudogene–lncRNA-mediated epigenetic silencing of OCT4. Performing mOct4P4 lncRNA pulldown experiments and a mOct4P4 lncRNA deletion analysis we demonstrate that the RNA binding protein FUS and a 200 nucleotide mOct4P4/hOCT4P3 region are essential for Oct4/OCT4 silencing in mouse and human cells. Binding of FUS to endogenous, full length mOct4P4/hOCT4P3 lncRNAs allows subsequent binding of SUV39H1 to the 200-nucleotide lncRNA element, forming a silencing complex with target specificity for the parental Oct4/OCT4 promoter. In experimental cell lines, the 200nt mOct4P4/hOCT4P3 lncRNA sequence element is sufficient to guide SUV39H1 dependent Oct4/OCT4 silencing, even in the absence of FUS.
We thus propose a model where FUS represents a licensing factor that mediates the accessibility of the 200 nucleotide mOct4P4/hOCT4P3 to SUV39H1 binding, thereby imposing target specificity of the silencing complex towards the parental Oct4/OCT4 gene promoter. Our data highlight the evolutionary relevance of pseudogene lncRNA mediated control of parental gene expression and the role of FUS in instructing the formation of an epigenetic regulatory complex with target site specificity defined by a lncRNA component.
Results
Conserved role of hOCT4P3 and mOct4P4 in silencing parental gene expression
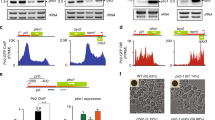
We recently demonstrated that the mouse mOct4P4 lncRNA–SUV39H1 complex targets conserved promoter elements of the ancestral Oct4 gene in trans, mediating gene silencing during mESC differentiation. To support the relevance of pseudogene lncRNA mediated epigenetic regulation of parental gene expression we tested whether this mechanism is conserved in human cells. To date, eight human POU5F1 pseudogenes have been annotated in the human genome25. Similar to mOct4P4, the human hOCT4P1, hOCT4P3, and hOCT4P4 pseudogenes have an exon structure that is similar to the OCT4 mRNA and show 81%, 82%, and 82% overall sequence identity to OCT4, respectively25. We previously showed that OCT4 is frequently expressed in ovarian cancer cell lines and controls cancer relevant pathways in OVCAR-3 cells15. This identifies OVCAR-3 ovarian cancer cells as ideal model system to validate conservation of pseudogene lncRNA mediated silencing of parental OCT4. hOCT4P3 lncRNA displays high sequence similarity to mOct4P4 and reproduces nuclear localization pattern in a series of human ovarian cancer cell lines (Fig. 1a, b)25.
a Schematic representation of murine mOct4P4 and human hOCT4P3 pseudogenes. Length of sequence elements and percentage of sequence homology are indicated. Gray boxes, sequences with homology to Oct4/OCT4 5′UTR; gray lines, sequences with homology to Oct4/OCT4 3′UTR. A centrally located, 334-bp spliced fragment is exclusively present in mOct4P4 (29). b Subcellular localization of hOCT4P3 in human Ovarian Cancer cell lines OVCAR-3, SKOV3, TOV-112D, and CAOV3 as determined by quantitative RT-PCR (qRT-PCR). Shown values refer to the percentage of total RNA expression. c Quantitative RT-PCR analysis of hOCT4P3 (left panel), OCT4 and pluripotency marker genes (right panel) in OVCAR-3 cells stably expressing hOCT4P3. Expression levels were normalized against ACTIN. d dCas9-HA-KRAB western blotting analysis (top) and RT PCR analysis (bottom) of Oct4 pseudogene guide RNA (sgOct4P4, sgOCT4P3) in mouse embryonic stem cells (mESCs) (left panel) and OVCAR-3 cells (right panel). ACTIN and Gapdh were used as control. e, f mOct4P4 lncRNA (e) and Oct4 (f) expression in self-renewing mESCs (EB T0) and during 10 days of embryoid body (EB) differentiation (EB D3–D10). Expression levels were normalized to Gapdh. g qRT-PCR analysis of self-renewal marker genes (left panel) or markers of early mESC differentiation (right panel) in dCas9/sgOct4P4 mESCs. Expression values were normalized against gapdh. h Percentage of contractile cardiomyocyte structures in embryoid bodies (EBs) obtained from dCas9 or dCas9/sgOct4P4 cells. i, j Quantitative RT-PCR showing hOCT4P3 lncRNA (i) and OCT4 (j) expression in dCas9 or dCas9/sgOCT4P3 OVCAR-3 cells. Expression values were normalized using ACTIN. k OCT4 expression in knockdown dCas9 and dCas9/sgOCT4P3 OVCAR-3 cells as determined by western blotting. ACTIN was used as control. Numbers represent OCT4/ACTIN ratio (dCAS9 empty was set “100”). l Chromatin immunoprecipitation (ChIP) analysis on the OCT4 promoter region in dCas9 and dCas9/sgOCT4P3 OVCAR-3 cells using H3K9me3 antibodies. Error bars represent standard deviation; Precise p values are indicated; n number of independent experiments carried out.
Stable overexpression of hOCT4P3 in OVCAR-3 cells leads to reduced OCT4 expression and downregulation of the self-renewal transcription factors SOX2, NANOG, and KLF4, indicative for impaired self-renewal circuits (Fig. 1c). Quantitative real-time polymerase chain reaction (RT-PCR) experiments revealed that hOCT4P3 and OCT4 transcript levels are 130- or 150-fold lower than the housekeeping gene DAXX. This indicates that, although present at low copy number, hOCT4P3 has an important role in parental gene expression control (Supplementary Fig. 1a). To demonstrate conservation of hOCT4P3 and mOct4P4 function we used the CRISPR/dCas9–HAKRAB system to silence hOCT4P3 or mOct4P4 lncRNA expression in OCVAR-3 or mESC cells, respectively.
We first generated mESC and human OVCAR-3 ovarian cancer cell lines stably expressing an HA-tagged version of a catalytically dead Cas9 version fused to the Kruppel associated box (dCas9-HAKRAB; dCas9 empty cells). In a subsequent step dCAS9 empty cells were stably transfected with an expression vector encoding short-guide RNAs (sgRNAs) that locate dCas9–HAKRAB to the promoter region of the Pou5f1P4/POU5F1P3 genes (dCAS9 sgOct4P4 mESCs or dCAS9 sgOCT4P3 OVCAR-3 cells). Expression of dCAS9-HAKRAB and respective sgRNAs in experimental mESCs and OVCAR-3 cells was validated by western blotting and RT-PCR (Fig. 1d). We previously demonstrated that mOct4P4 is efficiently upregulated during in vitro mESC differentiation17. Here, we used embryoid body (EB) differentiation as model system to address the impact of reduced mOct4P4 lncRNA expression on self-renewal and early differentiation markers. dCAS9 empty and dCAS9 sgOct4P4 mESCs were cultivated in hanging drop cultures in the absence of the self-renewal factor leukemia inhibitory factor (see “Methods”). We found that upregulation of mOct4P4 expression was strongly impaired during EB differentiation of dCAS9 sgOct4P4 mESCs (Fig. 1e). This effect was paralleled by inefficient Oct4/OCT4 silencing during 10 days of EB differentiation on the RNA and protein level (Fig. 1f, Supplementary Fig. 1b). Accordingly, we found increased expression of self-renewal transcription factors Sox2, Nanog, and Gdf3 and reduced expression of early differentiation markers Fgf5 and Nestin (Fig. 1g). On the functional level, dCAS9 sgOct4P4 embryoid bodies showed poor formation of contractile cardiomyocyte structures, indicative for in vitro differentiation defects (Fig. 1h, Supplementary Fig. 1c, Supplementary Movies 1 and 2). Importantly, reduced expression of human hOCT4P3 in dCAS9 sgOCT4P3 OVCAR-3 cells was paralleled by increased expression of OCT4 at the RNA and protein level (Fig. 1j, k). This effect was paralleled by reduced H3K9me3 at conserved elements at the promoter of the parental OCT4 gene (Fig. 1l). Based on our loss and gain of function experiment, we conclude that hOCT4P3 recapitulates mOct4P4 function in human OVCAR-3 cells. Importantly, data from dCAS9–HAKRAB loss of function models also demonstrate that endogenous mOCT4P4 and hOCT4P3 lncRNAs have a suppressive action on the Oct4/OCT4 promoter in mESCs and OVCAR-3 cells.
Our results demonstrate the evolutionary conservation of H3K9me3 dependent silencing of parental Oct4/OCT4 by mouse and human mOct4P4 and hOCT4P3 sense lncRNAs. This further implies the existence of defined lncRNA sequence elements essential for site specific targeting of SUV39H1 to the Oct4/OCT4 promoter.
A deletion analysis identifies mOct4P4 lncRNA regions essential for Oct4 silencing
The MS2 RNA tagging system enabled us to demonstrate that a mOct4P4 lncRNA–SUV39H1 complex locates to the promoter of the ancestral Oct4 gene in trans17. In order to identify lncRNA regions essential for mOct4P4 function we used a mESC cell line stably expressing a flag-tagged version of the MS2 phage coat protein (MS2-flag mESCs) as well as mOct4P4 deletion constructs that were tagged with 24 repeats of the MS2 RNA stem loop motif (Fig. 2a, Supplementary Fig. 2a). To ensure nuclear localization, ectopically expressed lncRNAs contained mOct4P4 regions corresponding to the 5′ and 3′ UTR regions of parental Oct4, previously shown to determine nuclear restriction of the endogenous mOct4P4 lncRNA (Fig. 2a)17.
a Schematic representation of the mOct4P4-24xMS2 deletion constructs. Gray boxes, sequences with homology to Oct4/OCT4 5′UTR; gray lines, sequences with homology to Oct4/OCT4 3′UTR; 334 bp lines represent centrally located, spliced fragment present in mOct4P4. 24xMS2 RNA stem loop motifs located at the 3′ end of mOct4P4 deletion constructs are indicated. Arrows indicate the locations of RT-PCR primers used to amplify mOct4P4 deletion constructs. b Levels of ectopic expression of mOct4P4-24xMS2 deletion construct (a) in mESCs, as determined by qRT-PCR. p Values relate to CTRL. c Subcellular localization of lncRNAs derived from mOct4P4-24xMS2 lncRNA deletion constructs (a) in mESCs, as determined by qRT-PCR. Expression values are shown as percentage of total RNA levels. p Values indicate significant nuclear versus cytoplasmic localization. d Oct4 mRNA levels in mESCs with ectopic expression of mOct4P4 deletion constructs (a), as determined by qRT-PCR. Expression values were normalized against gapdh. p Values relate to CTRL. e Representative image of OCT4 western blotting analysis in mESC cells overexpressing mOct4P4 deletion constructs shown in (a). ACTIN was used as loading control. Numbers represents values of OCT4 expression as mean of three independent experiments (control was set “100”). f, g anti-flag ChIP (f) and anti H3K9me3 ChIP (g) on the Oct4 promoter region in mESCs stably overexpressing 24xMS2 tagged deletion constructs shown in (a). qRT-PCR was performed to measure promoter enrichment; p values relate to MS2-flag/24xMS2-CTRL. Precise p values are indicated; n number of independent experiments carried out.
Established stable mESC cell lines displayed nuclear enrichment of all mOct4P4-24xMS2 lncRNA versions (Fig. 2b, c). Ectopic expression of full length mOct4P4-24xMS2 but also deletion constructs with 200 or 400 nucleotides deletions located at the mOct4P4 3′ terminus (Δ200, Δ400) efficiently reduced Oct4 mRNA levels, as determined by quantitative RT-PCR (Fig. 2d). Remarkably, constructs lacking 600 nucleotides or more extended 3′ lncRNA regions were no longer able to reduce Oct4 RNA expression (Δ600, Δ800, Δ994; 5′ + 3′; Fig. 2d). These results were recapitulated by western blotting using an OCT4 specific antibody (Fig. 2e). These data indicate that functionally relevant mOct4P4 sequence elements are anticipated to be located between position 984 and 1188 of the mature mOct4P4 lncRNA.
We next evaluated the ability of mOct4P4-24xMS2 deletion construct derived lncRNAs to (i) tether the flag-tagged MS2 phage coat protein to the Oct4 promoter and (ii) trigger increased H3K9me3 levels at the Oct4 promoter. Anti-flag ChIP experiments revealed that only MS2 RNA tagged full length, Δ200 and Δ400 Oct4P4-24xMS2 lncRNAs were able to locate the flag-tagged MS2 protein to the promoter of the ancestral Oct4 gene and to trigger a local increase of H3K9me3 (Fig. 2f, g, Supplementary Fig. 2b). Accordingly, MS2 RNA tagged Oct4P4 lncRNA versions that failed to suppress Oct4 expression (Δ600, Δ800, Δ994; 5′ + 3′; Fig. 2d, e) were unable to locate flag-tagged MS2 and H3K9me3 to the Oct4 promoter (Fig. 2f, g). Of notice, ectopically expressed full length mOct4P4-24xMS2 lncRNA was exclusively recruited to the Oct4 promoter but not to the promoters of Daxx, H2Q10, Ceher1, Pp1r18, and Rab5A genes that are localized up- and downstream of Oct4 on chromosome 17 (Supplementary Fig. 2c).
Together, this indicates that a 200 nucleotide sequence spanning position 984–1183 of the mOct4P4 lncRNA has a central role in orchestrating target site specific epigenetic silencing of the ancestral Oct4 gene in trans.
A 200 nucleotide mOct4P4 region is sufficient to silence parental gene expression
To directly test the importance of the 200 nucleotide mOct4P4 lncRNA region, we generated expression constructs encoding MS2 RNA-stem loop tagged mOct4P4 that lacks the relevant 200 nucleotide region (−200 bp -mOct4P4-24xMS2) but also a construct encoding a MS2 RNA motif tagged mOct4P4 lncRNA version that exclusively covers region 984–1183 (200 bp-mOct4P4-24xMS2) (Supplementary Fig. 2d). Both constructs contained the 5′ and 3′ mOct4P4 regions to ensure nuclear localization of expressed lncRNAs (Fig. 3a). Stable MS2-flag mESC clones expressed −200 bp-Oct4P4-24xMS2, 200 bp-Oct4P4-24xMS2, or full length mOct4P4-24xMS2 lncRNAs as nuclear restricted RNAs, as validated by quantitative RT-PCR (Fig. 3a–c).
a Schematic representation of generated mOct4P4-24xMS2 deletion constructs. Gray boxes, sequences with homology to Oct4/OCT4 5′UTR; gray lines, sequences with homology to Oct4/OCT4 3′UTR. 334 bp-spliced sequences are present in mOct4P4 and −200mOct4P4 sequences; deleted regions are indicated. White boxes indicate identified 200 nucleotide mOct4P4 region. 24xMS2 RNA stem loop motifs at the 3′ end of mOct4P4 deletion constructs are indicated. Arrows indicate the locations of RT-PCR primers. b qRT-PCR determining 200 bp-mOct4P4 and −200 bp-mOct4P4 expression levels in experimental mESCs. Expression values were normalized to Gapdh. c Subcellular localization of lncRNAs derived from constructs in (a). Expression values are shown as percentage of total RNA levels, as determined by qRT-PCR. d, e qRT-PCR (d) and western blot analysis (e) using mESCs ectopically expressing mOct4P4, 200 bp-mOct4P4, and −200 bp-mOct4P4 constructs. Oct4 expression values were normalized against Gapdh (d) or ACTIN (e). Shown numbers represent OCT4/ACTIN ratio as mean of three independent experiments (control was set “100”) (e). f, g ChIP analysis of Oct4 promoter region in mESCs stably overexpressing indicated constructs and using described antibodies. qRT-PCR was performed to measure promoter enrichment. Only mOct4P4 and 200 bp-mOct4P4 constructs localize to the Oct4 promoter (f) and drive H3K9me3 enrichment (g). Error bars represent standard deviation. Precise p values are indicated. n: number of independent experiments carried out.
We next tested the impact of ectopic lncRNA expression on H3K9me3 mediated silencing of parental Oct4. Quantitative RT-PCR and western blotting revealed that −200 bp-mOct4P4-24xMS2 expression failed to silence Oct4 expression. In contrast, expression of the 200bp-mOct4P4-24xMS2 lncRNA version mediated efficient suppression of ancestral Oct4, recapitulating silencing by full length mOct4P4-24xMS2 lncRNA (Fig. 3d, e). In line with this, ChIP experiments showed that the 200bp-mOct4P4-24xMS2 lncRNA, but not the -200bp-mOct4P4-24xMS2 - version recruited MS2-flag and H3K9me3 to the parental Oct4 promoter (Fig. 3f, g).
We conclude that mOct4P4 pseudogene lncRNA contains two regions with an essential role in silencing of the ancestral Oct4 gene: (i) 5′ and 3′ located sequences to ensure nuclear lncRNA and (ii) region 984–1183 that directs H3K9me3 to the Oct4 promoter.
FUS interacts with endogenous mOct4P4 to allow parental Oct4 gene silencing
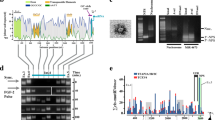
In order to obtain additional insights into the mechanism of mOct4P4 lncRNA mediated silencing of Oct4 we aimed to identify mOct4P4 lncRNA interacting proteins. MS2-flag cells expressing full-length mOct4P4-24xMS2 and control mESCs expressing only a 24xMS2 stem loop control RNA were used to perform anti-flag RNA immunoprecipitation (RIP) experiments. Obtained control and mOct4P4-24xMS2 RNA-immunoprecipitates where run on denaturing polyacrylamide gels. After Coomassie staining, protein bands specifically appearing in eluates from mOct4P4-24MS2 RIPs were cut out from the gel and subjected to mass spectrometry (Fig. 4a). Flag-tagged MS2 as well as an additional set of proteins were shown to be specifically over-represented in analyzed protein bands obtained from mOct4P4 lncRNA RIP eluates (Fig. 4a, Supplementary Table 1a, Supplementary Data 1). Given the reported involvement in gene silencing, we focused our interest on the RNA and DNA binding protein FUS28,29. In addition to transcriptional regulation, FUS has been demonstrated to be involved in DNA repair, alternative splicing, transcriptional regulation, RNA localization and stress granules30. FUS translocation events and mutations have been linked with liposarcoma and amyotrophic lateral sclerosis, respectively31,32,33.
a Silver stained protein gel of eluates obtained from mOct4P4-24xMS2 anti-flag RIP experiments. mESCs expressing flag-MS2 and mOct4P4-24xMS2 were used. Indicated bands specifically elute from mOct4P4-24xMS2 lncRNA. Protein identity was determined by mass spectrometry (Supplementary methods). b Anti-flag RIP using mESCs expressing MS2-flag/full length mOct4P4-24xMS2 or 24xMS2 RNA control cells using anti-flag antibody. Agarose gel electrophoresis after quantitative RT-PCR demonstrates the presence of mOct4P4-24xMS2 stem loop RNA (bottom panel). Detection of FUS and MS2-flag proteins by Western blotting (top and middle panel respectively). Bands analyzed by mass spectrometry are indicated as numbers (1–6); complete data on protein identification is available in the provided Supplementary Data 1. c Anti-FUS RIP using MS2-flag mESCs expressing full length mOct4P4-24xMS2 or 24xMS2 RNA control. Presence of FUS and flag-MS2 in eluates was validated by western blotting (top and middle panel respectively). Quantitative RT-PCR followed by agarose gel electrophoresis verified the presence of mOct4P4-24xMS2 in anti-FUS RIP experiments (bottom panel). d FUS and OCT4 western blotting using eluates from mOct4P4-24xMS2 or 24xMS2 mESCs transiently transfected with the indicated siRNAs. ACTIN was used as loading control. Numbers represent OCT4/ACTIN ratio as mean of three independent experiments (24xMS2-CTRL siCTRL was set “100”). e, f ChIP analysis of Oct4 promoter region using an anti-FUS antibody in control or FUS knockdown mESCs (e) or pMEFs (f). Eluates were analyzed by qRT-PCR. g Fus and mOct4P4 expression levels in pMEFs transiently transfected with indicated siRNAs, as determined by qRT-PCR. Expression levels were normalized to Gapdh. h, i qRT- PCR analysis using pMEF cells subjected to siRNA-mediated knockdown of mOct4P4 and Fus. Expression values for Oct4 (h) or self-renewal markers (i) were normalized against Gapdh. Error bars represent standard deviation. Precise p values are indicated. n number of independent experiments carried out.
Validation of RIP eluates by western blotting and RT-PCR confirmed interaction of FUS with the full length mOct4P4 lncRNA (Fig. 4b). We were also able to detect mOct4P4-24xMS2 lncRNA as well as MS2-flag protein in the eluates from anti-FUS RIP experiments, corroborating FUS–Oct4 pseudogene lncRNA interaction (Fig. 4c).
We were next interested in evaluating whether FUS is required for mOct4P4 lncRNA mediated silencing of Oct4. Transient knockdown of FUS abolished mOct4P4 function, thus rescuing OCT4 protein expression in mOct4P4-24xMS2 lncRNA overexpressing mESCs (Fig. 4d). In line with this, ChIP experiments revealed that FUS localizes to the Oct4 promoter in MS2-flag mESCs overexpressing mOct4P4-24xMS2 (Fig. 4e).
We previously showed that the mOct4P4 lncRNA is essential to maintain SUV39H1-dependent silencing of parental Oct4 in primary mouse embryonic fibroblasts (pMEFs), indicating that persistent localization of the mOct4P4 lncRNA at the Oct4 promoter is essential to maintain Oct4 silencing in differentiated cells17.
To test whether mOct4P4 lncRNA is required for the localization of FUS to the Oct4 promoter we performed ChIP experiments in mOct4P4 lncRNA knock-down pMEFs. Our results show that loss of endogenous mOct4P4 lncRNA displaced FUS from the Oct4 promoter in pMEFs (Fig. 4f). Accordingly, siRNA mediated depletion of FUS from pMEFs significantly increased Oct4 mRNA expression, recapitulating the effect of mOct4P4 knockdown on parental gene expression (Fig. 4g, h). This effect was paralleled by increased expression of self-renewal transcription factors Sox2, Nanog and Klf4 (Fig. 4i). We conclude that FUS is essential for the initiation and maintenance of mOct4P4 lncRNA mediated silencing of Oct4 in order to suppress self-renewal circuits in differentiated mouse cells.
FUS facilitates binding of SUV39H1 to the mOct4P4 lncRNA
mOct4P4 deletion constructs revealed that crucial regions for Oct4P4 function are limited to a 200 nucleotide region, spanning positions 984–1183 (Fig. 3a, d–g). To test whether this RNA region interacts with SUV39H1 or FUS, we performed anti-SUV39H1 and anti-FUS RIP experiments using MS2-flag mESC clones overexpressing full length mOct4P4-24xMS2, −200 bp-mOct4P4-24xMS2 or 200 bp-mOct4P4-24xMS2 constructs.
We found that the SUV39H1 protein co-immunoprecipitated with the full-length mOct4P4-24xMS2 and 200 bp-mOct4P4-24xMS2 lncRNAs, but not with −200 bp-mOct4P4-24xMS2 lncRNA (Fig. 5a). Interestingly, all types of ectopically expressed mOct4P4 lncRNAs versions bound FUS in RIP experiments, suggesting that FUS binds multiple mOct4P4 lncRNA regions (Fig. 5b). In contrast, mOct4P4–SUV39H1 interaction critically depends on the presence of the 200 nucleotide motif. Notably, we did not find evidence for direct interaction of SUV39H1 and FUS in co-immunoprecipitation assays (Supplementary Fig. 3a, b). In addition, we did not find SUV39H1 peptides in our mass spectrometry data from mOct4P4-24xMS2 lncRNA pull down experiments (Supplementary Data 1). This is in line with a lack of SUV39H1 in published data on the FUS interacting proteome34,35,36,37,38. We conclude that direct SUV39H1–FUS interaction is not a pre-requisite for silencing complex formation.
a Anti-SUV39H1 RIP in mESCs stably expressing mOct4P4-24xMS2, 200 bp-mOct4P4-24xMS2 or −200 bp-mOct4P4-24xMS2 lncRNAs. Agarose gel electrophoresis after mOct4P4 specific quantitative RT-PCR (top panel) and anti-SUV39H1 western blotting (bottom panel) on RIP eluates are shown. Anti-HA RIP was used as negative control. Arrow indicates primer dimers. b Anti-FUS RIP in mESCs stably expressing 200 bp-mOct4P4-24xMS2 or −200 bp-mOct4P4-24xMS2 lncRNAs. Agarose gel electrophoresis after mOct4P4 specific quantitative RT-PCR (top panel) and anti-FUS western blotting (bottom panel) on RIP eluates are shown. Anti-TUBULIN RIP was used as negative control. c Anti-FUS RIP in mESCs stably expressing mOct4P4-24xMS2 or 200 bp-mOct4P4-24xMS2 lncRNAs. Agarose gel electrophoresis after qRT-PCR confirmed FUS-Oct4P4 binding (top panel). Immunoprecipitation of FUS was validated by western blotting (bottom panel). d Anti-SUV39H1 RIP in Fus knockdown mOct4P4-24xMS2 and 200 bp-mOct4P4-24xMS2 mESCs. mOct4P4 specific qRT-PCR followed by agarose gel electrophoresis is shown (top panel). Presence of SUV39H1 in RIP eluates was validated by Western blotting (bottom panel). e, f Anti-SUV39H1 RIP (e) and anti-FUS RIP (f) in mESCs stably expressing mOct4P4-24xMS2 or 24xMS2. Agarose gel electrophoresis after Oct4 mRNA or mOCT4P4 specific qRT-PCR is shown (top panels). Anti-TUBULIN RIP was used as negative control. Immunoprecipitation of SUV39H1 (e) or FUS (f) was validated by Western blotting (bottom panels e and f).
To study the functional interplay between FUS, SUV39H1, and mOct4P4 in determining silencing complex function, we first performed anti-FUS RIP in Suv39h1 knockdown MS2-flag mESCs, stably overexpressing full-length mOct4P4-24xMS2 or 200 bp-mOct4P4-24xMS2 lncRNAs. We found that FUS interacts with the full length mOct4P4-24xMS2 but also the 200 bp-mOct4P4 lncRNA version in the presence and absence of SUV39H1 (Fig. 5c, Supplementary Fig. 3c). Thus, SUV39H1 is dispensable for FUS–mOct4P4 lncRNA interaction.
In a second step we transiently depleted FUS from experimental cells and performed anti-SUV39H1 RIP experiments followed by mOct4P4 specific RT-PCR. We found that loss of FUS abolishes SUV39H1 binding to the full length mOct4P4 lncRNA (Fig. 5d, Supplementary Fig. 3d). Strikingly, binding of SUV39H1 to the 200 bp-mOct4P4-MS2 lncRNA (mOct4P4 positions 984–1183) does not require FUS (Fig. 5d). This indicates that binding of FUS to the full-length mOct4P4 lncRNA plays an important role in providing access for SUV39H1 to the 200 nucleotide region. However, in the context of reduced lncRNA sequence complexity of the 200 bp-mOct4P4-MS2 construct, the critical 200 nucleotide region appears to be directly accessible to SUV39H1, rendering the action of FUS dispensable.
Oct4 mRNA and mOct4P4 lncRNA share high sequence identity levels, raising the question as to whether SUV39H1 and FUS may also interact with the endogenous Oct4 mRNA.
Importantly, RIP experiments using mESCs demonstrated that under our experimental conditions SUV39H1 and FUS display binding specificity towards mOct4P4 lncRNA but not Oct4 or other mRNAs such as Sox2, Nanog, Gapdh, or Actin (Fig. 5e, f).
This demonstrates that sequence degeneration after mOct4P4 pseudogene formation resulted in the formation of binding sites for FUS and SUV39H1, conferring a new biological function to the mOct4P4 lncRNA. On the mechanistic level, our data indicate that FUS has a critical role in supporting the interaction of SUV39H1 with full length mOct4P4 lncRNA, suggesting that FUS licenses the formation of a functional SUV39H1–mOct4P4 lncRNA complex in mESCs.
FUS mediates targeting of SUV39H1 by mOct4P4 lncRNA to the Oct4 promoter
We next wished to investigate how lncRNA:protein binding requirements translate into site specific targeting of a SUV39H1 containing silencing complex to the Oct4 promoter. We first validated whether FUS has a role in directing mOct4P4 lncRNA and SUV39H1 to the Oct4 promoter.
Anti-flag ChIP experiments using chromatin from flag-MS2 mESCs expressing MS2-RNA tagged mOct4P4 lncRNA variants demonstrate that transient knockdown of Fus abolishes the recruitment of full length mOct4P4-24xMS2 but also SUV39H1 to the promoter of the ancestral Oct4 gene (Fig. 6a, b). In line with this, ChIP revealed reduced H3K9me3 levels at the Oct4 promoter and increased OCT4 protein expression after siRNA mediated depletion of Fus from full length mOct4P4 lncRNA overexpressing mESCs (Fig. 6c). This demonstrates that FUS is essential for targeting the endogenous mOct4P4 –lncRNA–SUV39H1 complex to the Oct4 promoter.
a–c ChIP analysis of the Oct4 promoter region in control, mOct4P4-24xMS2 or 24xMS2 overexpressing mESC lines, transfected with the indicated siRNAs. Antibodies used for RIP are shown. Quantitative RT-PCR was performed to evaluate enrichment of markers at the Oct4 promoter. d, e ChIP analysis of Oct4 promoter region in 24xMS2-CTRL, mOct4P4-24xMS2, and 200 bp-mOct4P4-24xMS2 mESCs after siRNA mediated depletion of Fus. Quantitative RT-PCR was performed to measure abundance of flag-MS2 (d) and H3K9me3 (e) at the Oct4 promoter. f OCT4 expression levels in 24xMS2-CTRL, mOct4P4-24xMS2 and 200 bp-mOct4P4-24xMS2 mESCs after Fus knockdown. ACTIN was used as loading control. Numbers represent OCT4/ACTIN ratio (24xMS2-CTRL siCTRL was set “100”). g ChIP analysis of FUS abundance at the Oct4 promoter region in control, mOct4P4-24xMS2 or Suv39h1 knockdown mOct4P4-24xMS2 overexpressing mESC lines. h, i ChIP analysis of Oct4 promoter region in control, mOct4P4-24xMS2 and 200 bp-mOct4P4-24xMS2 mESCs after siRNA mediated depletion of Suv39h1. Quantitative RT-PCR was performed to measure enrichment of flag-MS2 (h) or H3K9me3 (i) at the Oct4 promoter. j, k Oct4 specific qRT-PCR (j) and OCT4 western blotting (k) of Suv39h1 knockdown control, mOct4P4-24xMS2 and 200 bp-mOct4P4-24xMS2 mESCs. RNA expression values were normalized against Gapdh; ACTIN was used as loading control in western blotting experiments. Numbers represents OCT4/ACTIN ratio (24xMS2-CTRL siCTRL was set “100”). Error bars represent standard deviation. Precise p values are indicated. n number of independent experiments carried out.
Importantly, performing anti-flag ChIP we found that siRNA mediated depletion of Fus does not impair the localization of the 200 bp-mOct4P4-24xMS2 lncRNA version to the Oct4 promoter of experimental mESCs (Fig. 6d). Accordingly, 200 bp-mOct4P4 overexpression results H3K9me3 enrichment at the Oct4 promoter and a reduction of OCT4 protein expression in control but also Fus knockdown mESCs (Fig. 6e, f). Thus, FUS is dispensable for parental Oct4 silencing in the context of the minimal sufficient 200 nucleotide mOct4P4 construct. However, in context of the increased sequence complexity of endogenous, full-length mOct4P4, FUS is essential to license the interaction between SUV39H1 and mOct4P4 to allow the formation of a silencing complex with Oct4 promoter target-specificity.
To further dissect requirements for Oct4 promoter targeting we evaluated the relevance of SUV39H1 for targeting FUS and mOct4P4 lncRNA to the parental Oct4 gene. Anti-FUS ChIP experiments revealed that siRNA mediated depletion of Suv39h1 delocalizes FUS from the Oct4 promoter in mESCs ectopically expressing full length mOct4P4 or the 200 bp-Oct4P4 lncRNA (Fig. 6g). Importantly, siRNA mediated knockdown of Suv39h1 abrogates the localization of full length mOct4P4-24xMS2 but also 200 bp-mOct4P4-24xMS2 lncRNA versions to the promoter of the ancestral Oct4 gene, as demonstrated by anti-flag ChIP. This effect was linked with impaired imposition of H3K9me3 to the Oct4 promoter and loss of parental Oct4 silencing in both experimental cell lines (Fig. 6h–k, Supplementary Fig. 4).
These data highlight that FUS is essential to instruct the loading of the repressive SUV39H1 HMTase to the critical 200 mOct4P4 lncRNA nucleotide region. This FUS dependent step is central to program target specificity of SUV39H1, towards the promoter of the parental Oct4 gene.
Functional conservation of a FUS–SUV39H1–OCT4 pseudogene lncRNA silencing complex
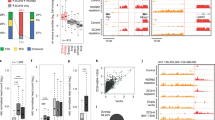
After identifying critical players for mOct4P4 function we set out to test whether all critical mechanistic steps are conserved in human OVCAR-3 cells. We first generated OVCAR-3 cell lines stably transfected with an expression vector encoding 24xMS2 tagged full-length hOCT4P3 (hOCT4P3-24xMS2) or a 24xMS2 tagged hOCT4P3 lncRNA region (200 bp-hOCT4P3-24xMS2) that corresponds to the functional relevant 200 nucleotide mOct4P4 region (Fig. 7a, Supplementary Fig. 5a). Functional experiments were carried out after transiently transfecting experimental cell lines with an expression vector encoding flag-tagged MS2.
a Schematic representation of the OCT4P3-24xMS2 and 200 bp-OCT4P3-24xMS2 constructs. Length of pseudogene segments are indicated. Gray boxes, sequences with homology to Oct4/OCT4 5′UTR; gray lines, sequences with homology to Oct4/OCT4 3′UTR. White boxes, 200 nucleotide hOCT4P3 region; 24xMS2 RNA stem loops aer indicated; arrows, position of RT-PCR primers. b Expression values of hOCT4P3 in hOCT4P3-24xMS2 OVCAR-3 cells, as determined by qRT-PCR. ACTIN was used as reference. c OCT4/OCT4 expression in 24xMS2-CTRL and hOCT4P3-24xMS2 OVCAR-3 cells, as determined by qRT-PCR (left panel) and western blotting (right panel). ACTIN/ACTIN was used to normalized expression values in qRT-PCR and as loading control in western blotting experiments, respectively. Numbers represent OCT4/ACTIN ratio (24xMS2-CTRL was set “100”). d Anti-flag RIP experiments in control and MS2-flag/hOCT4P3-24xMS2 OVCAR-3 cells. Top panel, agarose gel electrophoresis after hOCT4P3 specific, quantitative RT-PCR; bottom panel, western blotting of RIP eluates using anti-flag specific antibodies. e, f Evaluation of enrichment of flag-MS2 (e) and H3K9me3 (f) at the OCT4 promoter in control and hOCT4P3-24xMS2 OVCAR-3 cells by ChIP followed by quantitative RT-PCR. g, h Anti-flag RIP in control and hOCT4P3-24xMS2 OVCAR-3 cells. Top panels, western blotting of RIP eluates using the indicated antibodies. Bottom panel, agarose gel electrophoresis after hOCT4P3 specific, qRT-PCR. i Anti-SUV39H1 RIP in control and hOCT4P3-24xMS2 OVCAR-3 cells after siRNA mediated depletion of FUS. Top panel, hOCT4P3 specific qRT-PCR followed by agarose gel electrophoresis; bottom panel, anti-SUV39H1 western blotting using RIP eluates. j OCT4 and FUS expression in control and hOCT4P3-24xMS2 overexpressing OVCAR-3 cells after siRNA-mediated depletion of FUS, as determined by qRT-PCR (left panel) and western blotting (right panel). ACTIN was used as loading control. Numbers represent OCT4/ACTIN ratio as mean of three independent experiments (24xMS2-CTRL siCTRL was set “100”). k OCT4 expression in 24xMS2-CTRL, hOCT4P3-24xMS2 and 200 bp-hOCT4P3-24xMS2 OVCAR-3 cells as determined by qRT-PCR (left panel) and western blotting (right panel). ACTIN was used to normalized OCT4 expression values in qRT-PCR and western blotting experiments. Numbers represent OCT4/ACTIN ratio (24xMS2-CTRL was set “100”) (right panel). l OCT4 expression levels in 24xMS2-CTRL, OCT4P3-24xMS2, and 200 bp-hOCT4P3-24xMS2 OVCAR-3 cells under control or FUS knockdown condition, as determined by qRT-PCR (left panel) and western blot (right panel). ACTIN was used as loading control. Numbers represent OCT4/ACTIN ratio as mean of three independent experiments (24xMS2-CTRL siCTRL was set “100”). Error bars represent standard deviation. Precise p values are indicated. n number of independent experiments carried out.
In line with data from OVCAR-3 cells overexpressing untagged hOCT4P3 (Fig. 1c), we found that ectopic hOCT4P3-24xMS2 expression reduced the expression of endogenous OCT4/OCT4 on the RNA and protein level (Fig. 7b, c). Anti-flag RIP revealed interaction of MS2-flag with ectopically expressed hOCT4P3-24xMS2 lncRNA, as demonstrated by RT-PCR (Fig. 7d).
ChIP experiments using anti-flag and anti-H3K9me3 specific antibodies showed that the hOCT4P3-24xMS2 lncRNA localizes the flag-tagged MS2-protein to the promoter of the ancestral OCT4 gene, triggering a local increase in H3K9me3 (Fig. 7e, f). In line with this, western blotting and RT-PCR on protein and RNA fractions from anti-flag RIP eluates revealed that SUV39H1 and FUS co-immunoprecipitate with full length hOCT4P3-24xMS2 lncRNA (Fig. 7g, h).
Anti-SUV39H1 RIP experiments in control MS2-flag and MS2-flag/hOCT4P3-24xMS2 OVCAR-3 cells demonstrated that siRNA-mediated depletion of FUS disrupts binding of SUV39H1 to the full-length hOCT4P3 lncRNA (Fig. 7i, Supplementary Fig. 5b). In line with data from mESCs, transient depletion of FUS disrupts pseudogene lncRNA mediated reduction of OCT4 expression in full-length hOCT4P3-24xMS2 lncRNA overexpressing OVCAR-3 cells (Fig. 7j).
To validate the selective requirement of FUS for licencing full length OCT4 pseudogene lncRNA function in human cells we generated OVCAR-3 cells stably expressing a 24xMS2 tagged, 200 nucleotide hOCT4P3 region corresponding to the respective sequence stretch in the mOct4P4 lncRNA (Fig. 7a, Supplementary Fig. 5c). Importantly, we found that ectopic 200 bp-hOCT4P3 lncRNA expression recapitulates OCT4 silencing triggered by full length hOCT4P3 lncRNA (Fig. 7k). In line with data from mESCs, OCT4 silencing triggered by the 200 bp-hOCT4P3-24xMS2 lncRNA version was independent of FUS expression in OVACR-3 cells (Fig. 7l, Supplementary Fig. 5d, e).
We conclude that all aspects of mOct4P4 function are recapitulated by hOCT4P3 in human cells. This demonstrates that pseudogene lncRNA dependent silencing of Oct4/OCT4 represents an evolutionary conserved mechanism to fine-tune the expression of the parental Oct4/OCT4 gene.
On the mechanistic level we propose a model where FUS binding to the endogenous mOct4P4/hOCT4P3 lncRNA plays an important role in rendering the 200-nucleotide region accessible for SUV39H1 binding. This step is essential to license the formation of a SUV39H1 HMTase containing silencing complex with programmed target specificity towards the parental Oct4/OCT4 promoter (Fig. 8).
FUS binds to endogenous, full length mOct4P4/hOCT4P3 to provide access for SUV39H1 to 200 nucleotide lncRNA region (highlighted in green). Binding of mOct4P4/hOCT4P3 lncRNA to SUV39H1 creates a silencing complex with target specificity for the promoter of the ancestral Oct4/OCT4 leading to repression of parental Oct4/OCT4 by creating local H3K9me3 containing heterochromatin.
Discussion
Here, we investigate the molecular mechanism and evolutionary conservation of Oct4/OCT4 pseudogene lncRNA mediated control of parental gene expression. Repression of hOCT4P3 or mOct4P4 lncRNA expression in human OVCAR-3 or mESCs using the CRISPR/dCas9-HAKRAB system resulted in loss of H3K9me3 at the OCT4/Oct4 promoter and elevated OCT4/Oct4 expression levels (both at RNA and protein levels) in human or mouse cells, respectively. This indicates functional conservation of Oct4 pseudogene lncRNA mediated silencing of parental gene expression in mouse and human cells. High overall sequence identity and conservation of mOct4P4 function in human cells suggested the existence of functionally relevant lncRNA regions.
A deletion analysis identified a 200-nucleotide region in mOct4P4 and hOCT4P3 lncRNA that is required for targeting of the lncRNA-SUV39H1 silencing complex to the promoter of the ancestral Oct4/OCT4 gene, resulting in local H3K9 tri-methylation. Binding of Oct4/OCT4 pseudogene lncRNA by SUV39H1 is in line with studies demonstrating interaction of SUV39H1 HMTases with pericentric RNAs, telomere repeat containing RNA (TERRA), LINE1 L1MdA 5′UTR elements, SINE B1 repeats and pRNAs of the rRNA cluster39,40,41,42. Direct interaction of mOct4P4 lncRNA with SUV39H1 was recently demonstrated by in vitro EMSA experiments (37). SUV39H1 HMTase–RNA-binding specificity is reported to be promiscuous and characterized by low sequence specificity. This lead to the hypothesis that the formation of lncRNA–SUV39H HMTase complexes with defined epigenetic function may depend on additional proteins or the presence of physiologically functional RNA:chromatin templates43,44.
RNA pull-down experiments revealed a series of mOct4P4 lncRNA interacting proteins with a potential role in silencing parental Oct4. Here, we demonstrate that the RNA binding protein FUS has a critical role in Oct4/OCT4 lncRNA mediated silencing of OCT4. Loss of FUS prevents the formation of a full length mOct4P4/hOCT4P3 lncRNA–SUV39H1 silencing complex, abrogating the initiation and maintenance of Oct4/OCT4 silencing. Notable, FUS is dispensable for the function of the minimal sufficient mOct4P4/hOCT4P3 lncRNA version (200 bp-mOct4P4; 200 bp-hOCT4P3). Thus, we conclude that FUS does not have a central role in closing the Oct4/OCT4 promoter.
We propose that FUS is critical for the structuring the long Oct4 pseudogene lncRNA template to allow the binding of SUV39H1 to the 200-nucleotide region, thereby defining a specialized SUV39H1–lncRNA complex with selective target specificity towards the parental Oct4/OCT4 promoter. Importantly, FUS and SUV39H1 do not bind to the Oct4 mRNA in RIP experiments. This demonstrates that the specific interaction with FUS and the noncoding RNA-guided SUV39H1 HMTase represents a new biological feature of Oct4P4/OCT4P3 lncRNAs, that was acquired during pseudogene evolution. Future experiments will have to validate whether FUS has a more general role in epigenetic gene regulation by controlling the association of lncRNAs with epigenetic writers. In addition, the impact of Oct4/OCT4 promoter associated pseudogene transcripts on transcriptional initiation and Oct4/OCT4 promoter evasion remains an interesting issue to be addressed.
In contrast to the selective requirement of FUS for full length pseudogene lncRNA function, we found that SUV39H1 is essential for targeting of both, the full-length and 200 nucleotide mOct4P4/hOCT4P3 lncRNA versions to the Oct4/OCT4 promoter. Thus, after FUS dependent silencing complex formation, SUV39H1 and the 200 nucleotide mOct4P4/hOCT4P3 lncRNA regions hold the information for selective targeting and epigenetic silencing of the parental Oct4/OCT4 gene promoter.
The requirement of FUS as critical factor to license endogenous mOct4P4/hOCT4P3 lncRNA function may also represent a regulatory mechanism that restricts pseudogene-lncRNA mediated silencing to a defined biological context. Along these lines, PRMT1 dependent arginine methylation of FUS was recently shown to prevent the interaction with the CCND1 gene promoter-associated noncoding RNA-D (pncRNA-D), thereby blocking the repression of the HAT activity of the CBP/p300 HAT complex28,29. Addressing post-translational modifications of FUS may identify windows of mOct4P4/hOCT4P3 function in development and disease.
In addition to mOct4P4/hOCT4P3 also other pseudogene derived lncRNAs, such as DUXAP8 and DUXAP10 have been shown to interact with epigenetic writers12,45,46. However, DUXAP lncRNAs rather act as general scaffold for epigenetic regulatory complexes that do not selectively target the parental DUXA gene. In contrast, pseudogene PTENP1 antisense transcripts drive DNMT1 dependent silencing of the parental PTEN gene by paring with the 5′UTR of the nascent, sense PTEN RNA9,11. We experimentally validated that Oct4 and mOct4P4 are exclusively transcribed in sense orientation, thus excluding extended RNA:RNA interactions17. Thus, mOct4P4 and hOCT4P3 represent pseudogene sense lncRNAs that use a conserved mechanism to target and remodel the chromatin status of the parental gene promoter, located on a different chromosome.
Altogether, we propose a four-step model: (i) FUS binds mOct4P4/hOCT4P3 to (ii) allow SUV39H1 binding to the 200 nucleotide region, followed by (iii) sequence specific targeting of the Oct4/OCT4 promoter, resulting in (iv) increasing local H3K9me3 and HP1 levels and Oct4/OCT4 silencing (Fig. 8). The specific binding of SUV39H1 to H3K9me3 is anticipated to contribute to the maintenance of local heterochromatin structure at the Oct4/OCT4 promoter40,41.
Silencing of Oct4/OCT4 in trans may depend on complex long-range chromatin interaction of involved (pseudo)gene–loci, alternative DNA structures or the recruitment of additional factors. Elucidating mechanisms that functionally connect pseudogenes loci with ancestral genes will provide new insights into the power of pseudogenes encoded lncRNAs in fine-tuning the expression of ancestral genes in development and disease.
Methods
Cell culture
Feeder independent mESCs were cultured on 0.2% gelatin-coated plates using mESC self-renewal medium composed by Dulbecco’s modified Eagle’s medium (DMEM) (Lonza) supplemented with 15% knockout serum replacement (Gibco), 1% nonessential amino acids (Gibco), 1 mM sodium pyruvate (Gibco), 0.1 mM β-mercaptoethanol, 1% penicillin/streptomycin (Lonza) and 1000 U/ml mouse leukemia inhibitory factor47. OVCAR-3 cells were obtained from ATCC and cultured in RPMI-1640 medium (Lonza) supplemented with 20% (v/v) fetal bovine serum (FBS) (Lonza), insulin (10 μg/ml; I9278,Sigma) and 1% (v/v) penicillin/streptomycin (Lonza). Primary mouse embryonic fibroblasts (pMEFs) were generated in house from 13.5 d.p.c. C57BL/6 mouse embryos. pMEFs were maintained in culture in DMEM (Lonza) supplemented with 10% (v/v) FBS (Lonza) and 1% (v/v) penicillin/streptomycin (Lonza). Cell lines were maintained as monolayers at 37 °C in a humidified 5% CO2 atmosphere.
mESCs differentiation was obtained with a DMEM supplemented with 15% ES cell certified serum (Invitrogen), 1% non-essential amino acids (Gibco), 1 mM sodium pyruvate (Gibco), 1% l-glutamine (Invitrogen), 0.1 mM β-mercaptoethanol, and 1% penicillin/streptomycin (Invitrogen). EBs were generated by cultivating 300 cells in hanging drops culture for 3 days. Subsequently, EBs were transferred to a low-attachment 24-well plates (Euroclone) and grown in suspension for the indicated days. Alternatively, embryoid bodies were transferred to adherent cell culture dishes and cultivated for the indicated time periods to obtain contractile cardiomyocyte structures. All used cells were tested for mycoplasma contamination in regular intervals.
Viral transduction and generation of stable cell lines
Retroviral vectors such as pLPC-24xMS2, pLPC-mOct4P4-24xMS2, pLPC-mOct4P4-deletion constructs pLPC-200 bp-mOct4P4-24xMS2, pLPC-(−200 bp)-mOct4P4-24xMS2, pPLC-hOCT4P3-24xMS2, pLPC-200bp-hOCT4P3-24xMS2, and pMSCV-HA-MS2-Flag were packaged using 293GP cells. Forty-eight hours post transfection 10 ml of supernatants were harvested, filtered through a 0.45 µm filter and used to infect OVCAR-3 or ES cells in presence of polybrene. Twenty-four hours later medium was replaced with selection medium to obtain stable cell pools. Lentiviral vectors pLX-sgOCT4P3, pLX-sgOct4P4, and pHAGE-EF1α-dCas9-HA-KRAB (Addgene plasmid #50919) were packaged using 293T cells. Ten millilitre of supernatants were harvested, filtered through a 0.45 µm filter and used to infect OVCAR-3 or ES cells in presence of polybrene. Twenty-four hours later medium was replaced with selection medium to obtain stable cell pools. ES were transduced with retroviral vectors; OVCAR-3 cells with lentiviral and retroviral vectors. Cell lines infected with pLPC and pHAGE vectors were maintained in culture with 3 µg/mL of Puromycin and pMSCV and pLX vectors with 4 µg/mL of Blasticidine.
Transient transfection of plasmids and siRNAs
Transient transfections of plasmids were performed using TransIT®-LT1 transfection reagent (#MIR-2300, Mirus). Transient transfection of siRNA was performed using Lipofectamine RNAiMAX reagent (Invitrogen) according to manufacturer’s suggestions. Following siRNAs were used for transient siRNA experiments: Fus: GCAACAAAGCUACGGACAA (Eurofins Genomics); Suv39h1: CCAAUUACCUGGUGCAGAA (Thermo Scientific Dharmacon); mOct4P4 GAGCAUGAGUGGAGAGGAA (Thermo Scientific Dharmacon). Control siRNA was used as a negative control: Non-Targeting siRNA#1, TAGCGACTAAACACATCAA (Thermo Scientific Dharmacon).
RNA immunoprecipitation
Experimental cells were scraped in RIPA buffer (50 mM Tris-Cl, pH 7.5, 1% Nonidet P-40 (NP-40), 0.5% sodium deoxycholate, 0.05% sodium dodecyl sulfate, 1 mM EDTA, 150 mM NaCl) and supplemented with protease inhibitors (Complete, Roche) and RNaseOUT (Invitrogen). After incubation at 4 °C for 20 min, cell lysates were centrifuged. The supernatant was precleared for 1 h at 4 °C with protein A/G PLUS-Agarose (protein A/G PLUS-Agarose—sc-2003; Santa Cruz Biotechnology supplemented with yeast tRNA. 0.1 mg/mL). The precleared supernatant was incubated overnight at 4 °C with rabbit polyclonal anti-TLS/Fus (ab23439, abcam), mouse monoclonal anti-KMT1A/Suv39h1 (2.5 mg/ml, ab12405, Abcam) or mouse monoclonal anti-FLAG M2, clone M2 (2.5 mg/ml; F1804; Sigma) antibodies. RNA–protein complexes were recovered with protein A/G PLUS-Agarose beads and were washed six times in RIPA buffer. An aliquot of beads containing immunoprecipitated samples were saved for western blotting analysis. Remaining beads were used to obtain immunoprecipitated RNA that was analyzed by qRT-PCR. A mouse monoclonal anti-HA antibody, clone HA-7 (2.5 mg/ml, Sigma H9658) was used as negative control for immunoprecipitation.
Statistics and reproducibility
A one-tailed t test was performed to calculate p values and statistical significance was set at p < 0.05. Each finding was confirmed by three independent biological replicates, unless differently specified. Error bars represent standard deviation.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in this published article and related Supplementary information files. Source data of blots and gels are shown in Supplementary Fig. 6.
References
Frankish, A. & Harrow, J. Gencode pseudogenes. Methods Mol. Biol. 1167, 129–155 (2014).
Pei, B. et al. The GENCODE pseudogene resource. Genome Biol. 13, R51 (2012).
Poliseno, L. et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465, 1033–1038 (2010).
Cesana, M. et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147, 358–369 (2011).
Salmena, L., Poliseno, L., Tay, Y., Kats, L. & Pandolfi, P. P. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell 146, 353–358 (2011).
Tam, O. H. et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 453, 534–538 (2008).
Chiefari, E. et al. Pseudogene-mediated posttranscriptional silencing of HMGA1 can result in insulin resistance and type 2 diabetes. Nat. Commun. 1, 40 (2010).
Han, Y. J., Ma, S. F., Yourek, G., Park, Y. & Garcia, J. G. N. A transcribed pseudogene of MYLK promotes cell proliferation. FASEB J. 25, 2305–2312 (2011).
Johnsson, P. et al. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat. Struct. Mol. Biol. 20, 440–446 (2013).
Hawkins, P. G. & Morris, K. V. Transcriptional regulation of Oct4 by a long non-coding RNA antisense to Oct4-pseudogene 5. Transcription 1, 165–175 (2010).
Lister, N. et al. The molecular dynamics of long noncoding RNA control of transcription in PTEN and its pseudogene. Proc. Natl Acad. Sci. USA 114, 9942–9947 (2017).
Wei, C. C. et al. The pseudogene DUXAP10 promotes an aggressive phenotype through binding with LSD1 and repressing LATS2 and RRAD in non small cell lung cancer. Oncotarget 8, 5233–5246 (2017).
Tantin, D. Oct transcription factors in development and stem cells: Insights and mechanisms. Development 140, 2857–2866 (2013).
Chen, Z. et al. Clinicopathological significance of non-small cell lung cancer with high prevalence of Oct-4 tumor cells. J. Exp. Clin. Cancer Res. 31 (2012).
Comisso, E. et al. OCT4 controls mitotic stability and inactivates the RB tumor suppressor pathway to enhance ovarian cancer aggressiveness. Oncogene 36, 4253–4266 (2017).
Wang, L. et al. Pseudogene OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4 expression by competing for miR-145 in hepatocellular carcinoma. Carcinogenesis 34, 1773–1781 (2013).
Scarola, M. et al. Epigenetic silencing of Oct4 by a complex containing SUV39H1 and Oct4 pseudogene lncRNA. Nat. Commun. 6, 7631 (2015).
Gidekel, S., Pizov, G., Bergman, Y. & Pikarsky, E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell 4, 361–370 (2003).
Hochedlinger, K., Yamada, Y., Beard, C. & Jaenisch, R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121, 465–477 (2005).
Darini, C. Y. et al. Self-renewal gene tracking to identify tumour-initiating cells associated with metastatic potential. Oncogene 31, 2438–2449 (2012).
Koo, B. S. et al. Oct4 is a critical regulator of stemness in head and neck squamous carcinoma cells. Oncogene 34, 2317–2324 (2015).
Huang, P. et al. Implications of transcriptional factor, OCT-4, in human bladder malignancy and tumor recurrence. Med. Oncol. 29, 829–834 (2012).
Jóźwicki, W., Brożyna, A. A. & Siekiera, J. Expression of OCT4A: the first step to the next stage of urothelial bladder cancer progression. Int. J. Mol. Sci. 15, 16069–16082 (2014).
Suo, G. et al. Oct4 pseudogenes are transcribed in cancers. Biochem. Biophys. Res. Commun. 337, 1047–1051 (2005).
Poursani, E. M., Soltani, B. M. & Mowla, S. J. Differential expression of OCT4 pseudogenes in pluripotent and tumor cell lines. Cell J. 18, 28–36 (2016).
Xu, G., Yang, L., Zhang, W. & Wei, X. All the tested human somatic cells express both Oct4A and its pseudogenes but express Oct4A at much lower levels compared with its pseudogenes and human embryonic stem cells. Stem Cells Dev. 24, 1546–1557 (2015).
Bai, M. et al. OCT4 pseudogene 5 upregulates OCT4 expression to promote proliferation by competing with miR-145 in endometrial carcinoma. Oncol. Rep. 33, 1745–1752 (2015).
Wang, X. et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 454, 126–130 (2008).
Cui, W., Yoneda, R., Ueda, N. & Kurokawa, R. Arginine methylation of translocated in liposarcoma (TLS) inhibits its binding to long noncoding RNA, abrogating TLS-mediated repression of CBP/p300 activity. J. Biol. Chem. 293, 10937–10948 (2018).
Sama, R. R., Anjit, K., Ward, C. L. & Bosco, D. A. Functions of FUS/TLS from DNA repair to stress response: implications for ALS. ASN Neuro 6, 1759091414544472 (2014).
Mejzini, R. et al. ALS genetics, mechanisms, and therapeutics: where are we now? Front. Neurosci. 13, 1310 (2019).
Crozat, A., Åman, P., Mandahl, N. & Ron, D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature 363, 640–644 (1993).
Rabbitts, T. H., Forster, A., Larson, R. & Nathan, P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat. Genet. 4, 175–180 (1993).
Kamelgarn, M. et al. Proteomic analysis of FUS interacting proteins provides insights into FUS function and its role in ALS. Biochim. Biophys. Acta 1862, 2004–2014 (2016).
Chi, B. et al. Interactome analyses revealed that the U1 snRNP machinery overlaps extensively with the RNAP II machinery and contains multiple ALS/SMA-causative proteins. Sci. Rep. 8, 8755 (2018).
Kawaguchi, T. et al. Changes to the TDP-43 and FUS Interactomes Induced by DNA damage. J. Proteome Res. 19, 360–370 (2020).
Blokhuis, A. M. et al. Comparative interactomics analysis of different ALS-associated proteins identifies converging molecular pathways. Acta Neuropathol. 132, 175–196 (2016).
Reber, S. et al. The phase separation-dependent FUS interactome reveals nuclear and cytoplasmic function of liquid-liquid phase separation. bioRxiv https://doi.org/10.1101/806158 (2019).
Porro, A. et al. Functional characterization of the TERRA transcriptome at damaged telomeres. Nat. Commun. 5, 5379 (2014).
Johnson, W. L. et al. RNA-dependent stabilization of SUV39H1 at constitutive heterochromatin. Elife 6, e25299 (2017).
Camacho, O. V. et al. Major satellite repeat RNA stabilize heterochromatin retention of Suv39h enzymes by RNA-nucleosome association and RNA:DNA hybrid formation. Elife 6, e25293 (2017).
Schmitz, K. M., Mayer, C., Postepska, A. & Grummt, I. Interaction of noncoding RNA with the rDNA promoter mediates recruitment of DNMT3b and silencing of rRNA genes. Genes Dev. 24, 2264–2269 (2010).
Davidovich, C. et al. Toward a consensus on the binding specificity and promiscuity of PRC2 for RNA. Mol. Cell 57, 552–558 (2015).
Davidovich, C., Zheng, L., Goodrich, K. J. & Cech, T. R. Promiscuous RNA binding by polycomb repressive complex 2. Nat. Struct. Mol. Biol. 20, 1250–1257 (2013).
Guo, X., Lin, M., Rockowitz, S., Lachman, H. M. & Zheng, D. Characterization of human pseudogene-derived non-coding RNAs for functional potential. PLoS ONE 9, e93972 (2014).
Sun, M. et al. The pseudogene DUXAP8 promotes non-small-cell lung cancer cell proliferation and invasion by epigenetically silencing EGR1 and RHOB. Mol. Ther. 25, 739–751 (2017).
Murchison, E. P., Partridge, J. F., Tam, O. H., Cheloufi, S. & Hannon, G. J. Characterization of Dicer-deficient murine embryonic stem cells. Proc. Natl Acad. Sci. USA 102, 12135–12140 (2005).
Acknowledgements
M.S. and E.C. are supported by AIRC post-doctoral fellowships. M.R. is enrolled in the PhD program for Molecular Medicine at the University of Trieste. Financial support: This work was supported by AIRC grants (Rif 17756 to R.B., Rif 18381 to S.S., Rif. 22174, and Rif. 22759 to G.D.S.) and PRIN bando 2017 to G.D.S.
Author information
Authors and Affiliations
Contributions
M.S., S.S., and R.B. designed the experiments; M.S., E.C., and M.R. carried out the experiments; G.D.S. and C.S. supported the experimental design and data interpretation; S.S. and R.B. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scarola, M., Comisso, E., Rosso, M. et al. FUS-dependent loading of SUV39H1 to OCT4 pseudogene-lncRNA programs a silencing complex with OCT4 promoter specificity. Commun Biol 3, 632 (2020). https://doi.org/10.1038/s42003-020-01355-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-020-01355-9
This article is cited by
-
HOTAIR regulates SIRT3-mediated cardiomyocyte survival after myocardial ischemia/reperfusion by interacting with FUS
BMC Cardiovascular Disorders (2023)
-
Long non-coding RNAs: definitions, functions, challenges and recommendations
Nature Reviews Molecular Cell Biology (2023)
-
The roles of long noncoding RNAs in the regulation of OCT4 expression
Stem Cell Research & Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.