Abstract
The genetic and clinical characteristics of breast tumors with germline variants, including their association with biallelic inactivation through loss-of-heterozygosity (LOH) and second somatic mutations, remain elusive. We analyzed germline variants of 11 breast cancer susceptibility genes for 1,995 Japanese breast cancer patients, and identified 101 (5.1%) pathogenic variants, including 62 BRCA2 and 15 BRCA1 mutations. Genetic analysis of 64 BRCA1/2-mutated tumors including TCGA dataset tumors, revealed an association of biallelic inactivation with more extensive deletions, copy neutral LOH, gain with LOH and younger onset. Strikingly, TP53 and RB1 mutations were frequently observed in BRCA1- (94%) and BRCA2- (9.7%) mutated tumors with biallelic inactivation. Inactivation of TP53 and RB1 together with BRCA1 and BRCA2, respectively, involved LOH of chromosomes 17 and 13. Notably, BRCA1/2 tumors without biallelic inactivation were indistinguishable from those without germline variants. Our study highlights the heterogeneity and unique clonal selection pattern in breast cancers with germline variants.
Similar content being viewed by others
Introduction
Germline predisposition plays a substantial role in breast cancer, the most prevalent cancer in women. Management and prevention of breast cancer would, therefore, benefit from better knowledge and understanding of the genetic cause behind such familial predisposition1. Previous studies reported that pathogenic germline mutations account for 10.7% of breast cancer cases in a Western cohort2 and 9.2% of those in a Chinese cohort3. Breast cancer is also prevalent in Japan, affecting 116.3 per 100,000 women, where germline predisposition has been confirmed or suspected in as many as 5.7% of cases4. However, genetic studies of germline mutations that result in a predisposition to breast cancer are limited in the Japanese population4,5,6. In particular, the effects of pathogenic germline variants on somatic mutations and clinical/pathological phenotypes of accompanying breast cancers are poorly understood. Exception is the well-established mutational signatures associated with germline mutations in BRCA1/2 and PALB27,8, which are key genes in DNA repair by homologous recombination (HR) of DNA double strand breaks9. Although a previous study analyzed tumors with germline BRCA1/2 mutations in terms of presence or absence of biallelic inactivation10, its genetic and clinical impact on breast cancers have not been fully elucidated.
In this study, therefore, we investigated pathogenic germline variants in 11 genes implicated in hereditary breast cancer, which were BRCA1, BRCA2, TP53, PTEN, CDH1, STK11, NF1, PALB2, ATM, CHEK2, and NBN1,11,12,13,14,15,16,17,18, for 1995 unselected Japanese women with breast cancer, using targeted-capture sequencing of pooled DNA (Supplementary Fig. 1a). For those patients for whom tumor samples were available, the somatic alterations in the tumor were also interrogated in order to link the genetic features of the germline risk alleles to the associated tumor clinical presentations. In particular, we investigated the effects of biallelic inactivation of BRCA1/2 genes on the somatic mutations, and copy number (CN) abnormalities (CNAs) and clinical features of the resulting breast cancers.
Results
Prevalence of germline mutations
The characteristics of the study participants are shown in Table 1. Of the 1995 patients analyzed, 101 (5.1%) carried pathogenic germline variants, comprising 54 (53.5%) frameshift insertion or deletions (indels), 35 (34.7%) nonsense mutations, 7 (6.9%) missense mutations, 4 (4.0%) splice site mutations, and 1 (1.0%) synonymous mutation (Supplementary Fig. 1b). With respect to the 11 genes of interest, 77 of the total (3.9%) patients carried a variant in BRCA2 (n = 62, 3.1%) or BRCA1 gene (n = 15, 0.8%), followed by PALB2 (n = 9, 0.5%), TP53 (n = 4, 0.2%), PTEN (n = 4, 0.2%), CHEK2 (n = 3, 0.2%), ATM (n = 3, 0.2%), and NF1 (n = 1, 0.05%) (Fig. 1a, Supplementary Table 1). No pathogenic variants were identified in CDH1, STK11, and NBN. None of the patients carried two or more pathogenic variants. The genetic mutations were widely distributed along the entire coding region of the genes, except for missense mutations on the DNA binding domain of TP53 (Supplementary Fig. 2). Some variants were detected in ≥2 patients, such as L63X in BRCA119 and c.5576_5579del in BRCA23,4, which have been previously suggested as founder mutations in the Asian population19,20.
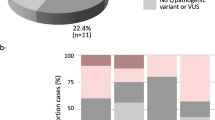
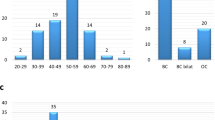
a Numbers and types of germline variants identified in each gene. b Box-and-whisker plots of age at breast cancer diagnosis for tumors with germline variants (n = 101), including BRCA1 (n = 15), BRCA2 (n = 62), and others (n = 24), and those without (n = 1892). The boxes indicating median and interquartile range, and the whiskers denoting the range. Asterisks indicate significance difference (Mann–Whitney U test: P < 0.05). c Distribution of phenotype according to the status of germline mutations. DCIS ductal carcinoma in situ, TNBC triple-negative breast cancer, LumHER2 luminalHer2. d Distribution of t factor for tumors according to the status of germline mutations. Tis carcinoma in situ.
Characteristics of patients carrying germline variants
The profiles of patients carrying germline variants in each gene are shown in Supplementary Table 2. Pathogenic variants were more frequently identified in patients with a family history of breast cancer (n = 41, 11.0%), compared with those without (n = 50, 3.4%) (P < 0.00001). Of the analyzed genes, BRCA2 was the most frequently mutated in both patients with and without a family history (Supplementary Table 3). A quarter of the patients with germline variants did not fulfill the NCCN criteria21 for assessment as high-risk for genetic or familial cancers.
The median age at diagnosis of patients with pathogenic germline variants was 53 years, which was younger than that of patients with no pathogenic variants (60 years) (P < 0.00001) (Fig. 1b, Supplementary Fig. 3a). BRCA1 germline mutations were associated with younger age at diagnosis (median age, 43 years), compared with BRCA2 (median age, 56 years; P = 0.08), and other 6 genes (median age, 52 years; P = 0.08). Among early onset breast cancer patients who were diagnosed before 35 years of age, prevalence of germline variants in BRCA1, BRCA2, and other genes was 9.8%, 17.1%, and 4.9%, respectively (Supplementary Fig. 3b).
As previously reported3, BRCA1 variant carriers were more likely to have triple-negative (estrogen receptor (ER) negative, progesterone receptor (PR) negative, and HER2 negative) diseases, compared with those with other germline variants (P = 0.0007) and those without germline mutations (P = 0.0001) (Fig. 1c). In contrast, there was no obvious tumor subtype associated with BRCA2 variant carriers. Advanced (T2–T4) breast cancers were more common in BRCA1-mutated cases, compared with those with pathogenic germline mutations in other genes (P = 0.08) or those with no pathogenic variants detected (P = 0.05) (Fig. 1d), especially among younger patients (Supplementary Fig. 3c). Lymph node involvement did not significantly correlate with the status of pathogenic germline variants (Supplementary Fig. 3d). Survival data were available for 1387 (69.5%) of 1995 patients with a median follow-up of 3 years (range: 0–35 years). No prognostic impact of germline mutations was demonstrated for overall and disease-free survival in both univariate and multivariate analyses (Supplementary Fig. 4, Supplementary Table 4).
Somatic alterations in tumors with germline BRCA1 and BRCA2 variants
Tumor samples were obtained from 30 patients with pathogenic germline variants in BRCA2 (n = 25) and BRCA1 (n = 5), as well as from an additional 30 patients without pathogenic germline mutations. Somatic mutations in common breast cancer drivers and CNAs were analyzed for these samples using targeted panel sequencing (Supplementary Table 5). In total, 19 of 30 samples with germline variants in BRCA1/2 had one or more somatic mutations in 18 driver genes with a median of 1 mutation/sample, which was significantly smaller than those without germline variants (median 2 mutations/sample, P = 0.004) (Fig. 2a, Supplementary Table 6). For tumors with germline BRCA1/2 mutations, somatic mutations were most frequently detected in PIK3CA (n = 6), TP53 (n = 6) and KMT2C (n = 6) (Fig. 2b, Supplementary Table 7). All samples had CNAs, regardless of the presence or absence of a pathogenic germline mutation. Even though the two-hit hypothesis of tumorigenesis predicts that majority of cases will have biallelic inactivation of the relevant cancer predisposing loci, biallelic inactivation of the predisposing alleles was found in only 20 cases (67%), while the remaining 10 retained an intact allele (mono-allelic inactivation). For each of the 20 cases, biallelic inactivation was caused by loss-of-heterozygosity (LOH) affecting the relevant germline variant loci. Indeed, nearly all cases involved deletions in BRCA2 (17/17) and BRCA1 (1/3), followed by copy-neutral LOH (uniparental disomy) (n = 1) and gain with LOH (n = 1). These results suggest that LOH is the predominant mechanism of biallelic inactivation of BRCA1/2 genes. Interestingly, one tumor with a germline BRCA2 variant also harbored a low allele frequency of somatic BRCA2 mutation in addition to LOH, suggesting clonal heterogeneity in the tumor over time, with independent events leading to biallelic inactivation.
a Numbers of driver mutations in tumors with germline variants in BRCA1/2 (n = 30) and those without (n = 30). Asterisks indicate significance difference (Mann–Whitney U test: P < 0.05). b The landscape of somatic variants in tumors with germline variants in BRCA1/2 (n = 30) and those without (n = 30). Distribution and types of recurrently mutated genes in tumors with germline BRCA1/2 mutations are shown. c Genome-wide CN changes in tumors with germline variants in BRCA1/2. Red: gain, dark red: gain with LOH, green: copy neutral LOH, blue: deletion. d Representative CN plots of tumors with and without biallelic inactivation. Regions with LOH are shown in orange lines. CN copy number, AsCN allele specific CN.
Mono-allelic vs. biallelic inactivation of BRCA1/2
BRCA1/2 genes normally function in DNA repair and their deleterious mutation has been linked to HR deficiency. Hence, in our evaluation of tumors with mono-allelic and biallelic inactivation of BRCA1/2, we first analyzed CNAs (Fig. 2c). As seen for representative cases in Fig. 2d, samples with mono-allelic BRCA1/2 lesions mainly showed CN gains with rare deletions. In contrast, biallelic BRCA1 inactivation showed extensive gains with or without LOH, and those with biallelic BRCA2 inactivation were characterized by extensive deletions or copy neutral LOHs. Although no specific patterns of CNAs have been reported in breast cancers with biallelic BRCA1/2 inactivation, these observations led us to hypothesize that specific genomic rearrangements caused by HR deficiency could cause characteristic CN changes. Combining the Japanese cases with 34 cases from TCGA breast cancer cases, we tested this hypothesis using a total of 64 cases with germline BRCA1/BRCA2 variants. In total, biallelic inactivation of BRCA1/2 loci was observed in 49 of 64 (77%) patients: 18/21 (86%) cases with BRCA1 and 31/43 (72%) cases with BRCA2 germline mutations (Fig. 3a). Tumors with mono-allelic BRCA1 and BRCA2 mutations are less common in the TCGA cases, compared with the Japanese cases, particularly those with mono-allelic BRCA1 mutations (1/16 vs. 2/5 for BRCA1 and 4/18 vs. 8/25 for BRCA2). The predominant role of LOH was also confirmed in TCGA cases, in which LOH explained 93% of biallelic inactivation in both BRCA1- and BRCA2-mutated cases, whereas biallelic inactivation via compound germline and somatic mutations were found only in two cases.
a Types of germline variants in BRCA1/2 according to the status of biallelic inactivation (n = 64). b Fraction of genomic regions with LOH in tumors without germline variants (n = 30) and in those harboring germline BRCA1 mutations with (n = 18) and without (n = 3) biallelic inactivation and those with germline BRCA2 mutations with (n = 31) and without (n = 12) biallelic inactivation. Asterisks indicate significance difference (Mann–Whitney U test: P < 0.05). c Frequency of TP53 mutations in tumors without germline variants (n = 30) and those harboring germline BRCA1/2 mutations with and without biallelic inactivation (n = 64). Asterisks indicate a significance difference (Fisher test: P < 0.05). d LOHs on chromosome 17 in patients with germline BRCA1 mutations (n = 17). LOHs identified in a single patient are shown on a line. e LOHs on chromosome 13 in patients with germline BRCA2 mutations (n = 3).
We next investigated characteristic patterns of mutations and structural variants (SVs) associated with biallelic BRCA1 or BRCA2 inactivation, focusing on mutational signatures and SVs using whole-exome sequencing (WES) data in the TCGA cohort. Four predominant mutational signatures were identified using pmsignature22 (Supplementary Fig. 5a). Of these, the mutational signature caused by deficient HR (Sig_3) was more frequent in tumors with biallelic BRCA2 inactivation than those without germline mutations, which concurs with previous reports7,8 (Supplementary Fig. 5b, c). In analysis of SVs for tumors with biallelic inactivation of BRCA1 and BRCA2, compared to tumors without germline variants, increased occurrence of tandem duplications and deletions (for BRCA1 inactivation) and deletions (for BRCA2 inactivation) were observed (Supplementary Fig. 5d, e). By contrast, tumors with mono-allelic inactivation of either BRCA gene did not show an increase in Sig_3 mutations and deletions/tandem duplications. In addition, tumors with biallelic BRCA2 inactivation exhibited significantly more extensive LOH lesions compared with those with mono-allelic inactivation and those without germline variants (Fig. 3b). Tumors with biallelic BRCA1 inactivation also tended to have more extensive LOH than those without germline variants, but a comparison with mono-allelic and biallelic BRCA1 inactivation was inconclusive due to the small number of patients with tumors of this mono-allelic category. These results suggest that biallelic BRCA1/2 inactivation causes extensive CNAs, in addition to small SVs.
Strikingly, except for one case, which displayed compound germline and somatic BRCA1 mutations, all but one tumors with biallelic BRCA1 inactivation (17/18) harbored TP53 mutations (Fig. 3c, Supplementary Fig. 5f). The TP53 mutations were accompanied by high variant allele frequency and loss of an intact chromosome 17, leading to biallelic TP53 inactivation (Fig. 3d, Supplementary Fig. 6a). Of added interest in this regard is the observation that some tumors with biallelic BRCA2 inactivation, commonly accompanied by LOH of chromosome 13, also exhibited concomitant biallelic inactivation of RB1, which was mutated in 3 cases (Fig. 3e, Supplementary Fig. 6b). RB1 mutations were more frequent in tumors with biallelic loss of BRCA2 (3/31, 9.7%) than those without (22/858 cases in our cohort and TCGA dataset, 2.6%) (P = 0.05). These results suggest that loss of chromosomes 17 and 13 play an important role in the development of breast cancer in patients with mutated BRCA1 and BRCA2, though inactivating TP53 and RB1, respectively. However, the number of tumors identified in our study that exhibited RB1 mutation in addition to biallelic loss of BRCA2 is small (n = 3), and further studies are warranted to confirm the association of these genetic lesions.
Finally, we evaluated the clinical characteristics of patients with mono-allelic and biallelic BRCA1/2 inactivation. Patients with biallelic inactivation showed a significantly younger onset than those without (median age at diagnosis: 47 vs. 59.6 years) (P = 0.01) (Fig. 4a), with no significant difference between patients with biallelic BRCA1 and BRCA2 inactivation. Although not significant, tumors having biallelic BRCA1 inactivation tended to have more advanced (T2–T4) (P = 0.09) and triple-negative breast cancer (P = 0.55) (Fig. 4b, c, Supplementary Table 8). We also analyzed tumors with germline BRCA1 and BRCA2 variants for classification into PAM50 gene expression subtypes using TCGA samples. In accordance with previous reports, tumors with biallelic BRCA1 inactivation were more frequently classified as basal-type23,24, compared to those without (Fig. 4d). In strong contrast to biallelic BRCA1/2 inactivation, samples with mono-allelic BRCA1/2 inactivation were not associated with younger age at onset, or an increase in triple-negative or basal-type tumors (Supplementary Table 8). The mutation status of BRCA1/2 or the presence or absence of biallelic involvement of these genes did not affected overall or disease-free survival both in univariate and multivariate regression analyses (Supplementary Fig. 7, Supplementary Table 9).
a Age at diagnosis of breast cancers harboring germline BRCA1/2 mutations with (n = 49) and without (n = 15) biallelic inactivation. The asterisk indicates significance difference (Mann–Whitney U test: P < 0.05). b–d Distribution of T factor: (b), in subtypes based on immunohistochemistry (c) in PAM50 mRNA-expression subtypes (d) of breast cancers harboring germline BRCA1/2 variants with and without biallelic inactivation. The difference in frequency of advanced (T2–T4) tumors, triple-negative breast cancer (TNBC), and basal-type tumors between tumors with and without biallelic inactivation were tested by the two-sided Fisher’s exact test. Tis carcinoma in situ, LumHER2 luminalHer2, LumA luminalA, LumB luminalB.
Discussion
In the current study, pathogenic germline mutations were detected in 5.1% of 1995 unselected Japanese breast cancer patients, which was equivalent to the frequency in the previous report of Japanese cohort4. The incidence rate of BRCA1/2 variants was also relatively similar with those reported in Japanese patients4 and Chinese population3. Given that the current study was restricted to detection of SVs and other variants that are not registered in ClinVar, the actual prevalence of pathogenic germline variants might be underestimated. Importantly, a half of the cases (50/101) was negative for a family history of breast cancer, or did not fulfill the NCCN criteria for high-risk of familial cancer, indicating the importance of investigating germline DNA, even among sporadic breast cancer patients.
In line with the previous reports8,10,25, tumors with mono-allelic BRCA1 and BRCA2 mutations were frequently observed in our cohort. Tumors with biallelic BRCA1/2 inactivation had unique genetic features, in terms of CNA and BRCA-associated mutational signature and SVs, which were not seen in those with mono-allelic inactivation. Although the only significant clinical difference between tumors with mono-allelic and biallelic BRCA1/2 inactivation was age at onset, the frequency of advanced stage, triple-negative or basal tumors tended to be higher in tumors with biallelic inactivation. The correspondence of biallelic BRCA1/2 inactivation with earlier age of onset conflicts with results of a previous study10. Although both studies included TCGA samples, here, we carefully removed low quality samples and whole genome-amplified samples from the analysis. Furthermore, we considered compound germline and somatic mutations of BRCA1/2 as contributors to biallelic inactivation, which likely increased the sensitivity of the dataset to detection of a correlation between the status of BRCA1 and BRCA2 and the clinical variables (Supplementary Fig. 8).
Tumors with mono-allelic BRCA2 mutations and those without BRCA1/BRCA2 mutations did not differ in their clinical presentation or analyses of additional genetic effects. In particular, mono-allelic tumors did not show an enhanced BRCA-related mutational signature or increase in SVs, which was seen in tumors with biallelic BRCA1/2 inactivation. Nevertheless, mono-allelic germline BRCA1/2 mutations show a significant enrichment in breast cancer patients, compared with the general population. Despite having only sequenced a portion of the tumors with BRCA1/2 variants in our cohort (30/77), a mono-allelic loss-of-function mutation in BRCA1 and BRCA2 was more frequent than within the control cohort4: ≥2/1995 (0.1%) vs. 5/11,241 (0.04%) for BRCA1 and ≥8/1995 (0.4%) vs. 15/11,241 (0.13%) for BRCA2. Thus, mono-allelic mutation alone does seem to play a role in the development of breast cancer, which is supported by several biological studies showing the effects of haploinsufficiency of BRCA1/2 in carcinogenesis26,27. This raises the question as to whether or not platinum28 or poly (ADP-ribose) polymerase (PARP) inhibitor29 are also effective against tumors with mono-allelic mutations. A lower sensitivity of PARP inhibitors to cells with heterozygous BRCA mutations have indeed been reported using in vitro30 and mouse models31. In contrast to this, however, Jonsson et al.32 reported that the allelic status did not affect the response of in tumors with germline BRCA1/2 mutations to PARP inhibitors32. Further evaluation of these drugs in the context of biallelic inactivation, is required in the future, incorporating clinical follow-up of patients, or clinical trials.
Finally, our study has revealed an intriguing linked mechanism of biallelic inactivation of TP53 with BRCA1 and RB1 with BRCA2, respectively. Tumors showing biallelic inactivation of both BRCA1 and TP53 genes were almost invariably associated with loss of normal chromosome 17. Of interest, a previous study using fluorescence in situ hybridization (FISH) and immunohistochemistry of TP53 on tumors with biallelic inactivation of both BRCA1 and TP53 have demonstrated that TP53 mutation occurs before LOH of the intact chromosome 17 as there remained cells with two chromosome 17 alleles and mutated TP53. The subsequent biallelic BRCA1 inactivation by LOH thus leads to biallelic inactivation of both TP53 and BRCA1 simultaneously25. A similar scenario is suggested here for RB1 and BRCA2 on chromosome 13, leading to simultaneous biallelic inactivation of the two genes via a deletion of part of chromosome 13.
In summary, we revealed that breast tumors with pathogenic germline BRCA1/2 variants show different genetic and clinical characteristics depending on the presence or absence of biallelic inactivation of these genes. Along with the recent data of different impact of mono-allelic and biallelic somatic TP53 mutations in myelodysplastic syndromes33, our data highlights the importance of allelic status of cancer driver genes.
Methods
Patients and samples
A total of 2136 breast cancer patients were enrolled in this study, treated between September 2011 and October 2016 at Kyoto Breast Cancer Research Network institutions, consisting of Kyoto University Hospital and 17 affiliated institutions. Among these, 1995 cases fulfilled the following inclusion criteria (Supplementary Fig. 1a): female; sufficient amount of high quality genomic DNA; pathological diagnosis of breast cancer; clinical data of at least one from age of onset, histology, phenotype, grade, clinical stage, past history, and family history.
These cases were collected consecutively with no selection bias. Family history was defined as the presence of one or more first- or second-degree relatives with breast and/or ovarian cancer. ER, PR, and HER2 status was determined by immunohistochemistry and/or FISH using breast tumor tissue obtained from a core needle biopsy or taken during surgery. For HER2 status, an immunohistochemistry score of 0 and 1+ was considered negative, whereas 3+ was considered positive. Tumors with a score of 2+ were evaluated further by FISH.
Written informed consent was obtained from all participants. The study was reviewed and approved by the Ethics Committees of Kyoto University Graduate School and Faculty of Medicine and Kyoto University Hospital and was performed in accordance with the Declaration of Helsinki (2013 revision).
Targeted sequencing of pooled DNA samples
Genomic DNA samples were extracted from peripheral blood samples of patients using a Gentra Puregene Kit (Qiagen). After adjusting the concentration of each genomic DNA sample to 50 ng/μL, each sample from 10 to 20 consecutive patient extractions were combined into one DNA pool34, generating a total of 106 DNA pools. Pooled DNA samples were analyzed using targeted-capture sequencing of 11 genes implicated in hereditary breast cancer using the SureSelect Custom kit (Agilent Technologies). RNA probes were designed to cover all coding regions and intron–exon boundaries of the 11 breast cancer susceptibility genes. Captured libraries were sequenced on a HiSeq 2000 (Illumina). To confirm the sensitivity of pooled sequencing, we first generated 2 pools from 40 patients. These 40 patients were individually genotyped and 60 private germline variants in 11 genes were detected. Next, 2 pools were sequenced at 1436–1476×, and 58/60 (97%) of variants were of variant allele frequency (VAF) > 0.005. Subsequently, 106 DNA pools were sequenced. The mean coverage of pooled DNA samples was 1644× (1267×–2316×). Reads were aligned to the reference human genome GRCh37. Germline variations were called using EBCall (Empirical Bayesian mutation Calling, https://github.com/friend1ws/EBCall)35,36 with for following parameters: (i) a variant allele frequency (VAF) ≥ 0.005; (ii) a P value < 0.01 (by EBCall); (iii) removal of SNPs in ESP, the 1000 genomes project, ExAC and HGVD (http://www.genome.med.kyoto-u.ac.jp/SnpDB/) with a minor allele frequency of ≥0.001; (iv) support from ≥2 reads. Variants were annotated using Annovar (http://annovar.openbioinformatics.org/en/latest/). Three BRCA1/2 mutations already detected in clinical purposes were successfully identified. For each pool with positive variant calls, the variant-positive samples were interrogated by amplicon-sequencing or Sanger sequencing of all samples in the corresponding pool with germline variants. Finally, 101 out of 117 germline mutations were validated. High allele frequencies of variants were supported by both of sequencing chromatograms and the VAFs of amplicon sequencing. Except for the long (20 bp) deletion variants, the VAFs of deep sequencing were ≥0.33, supporting the germline nature of these mutations.
Variant classification of germline variants
Truncating mutations (nonsense mutations or frameshift indels) were considered as pathogenic, except for low-risk truncating mutations, such as the K3326X mutations of BRCA2. Missense, synonymous and splice site mutations registered as “pathogenic” or “likely pathogenic” in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/)37,38 were also considered as pathogenic variants in this study (Supplementary Fig. 1b).
Targeted sequencing of tumor samples
To identify and characterize somatic mutations in tumors from patients with germline variants in BRCA1/2, 36 tumor samples with germline variants in BRCA1/2 and those without pathogenic germline mutations (n = 35) were analyzed by target sequencing using a SureSelect system (Agilent). All the tumor samples were collected prior to treatment. For formalin-fixed paraffin-embedded (FFPE) samples, a KAPA Hyper Prep Kit (KAPA Biosystems, Wilmington, MA) was also used before target enrichment. RNA probes were designed to capture 115 genes associated with breast cancer (Supplementary Table 5) and 1275 SNP sites for the measurement of genomic CNs. Based on the allele frequency of mutations and CN changes, we excluded 11 samples with a lower tumor cell fraction from further analysis. Finally, 30 samples with germline variants in BRCA1/2, including frozen tissues (n = 5) or FFPE samples (n = 25), and 30 tumors without germline mutations were analyzed. The mean coverage of fresh frozen and FFPE samples were 599× (347×–1253×) and 293× (112×–557×), respectively. Somatic mutations were analyzed using EBCall, with the following parameters: (i) removal of SNPs in ESP, the 1000 genomes project, ExAC and HGVD with a minor allele frequency of ≥0.001; (ii) support from ≥5 reads in the tumor; (iii) a VAF ≥ 0.02; (iv) a P value < 0.001 (by EBCall); (v) support from reads mapped to both strands. Variants with a VAF ≥ 0.4 were removed as germline SNPs, except for loss-of-function mutations in tumor suppressor genes and gain-of-function mutations reported in the COSMIC database. Synonymous variants were also excluded as germline variants. Mapping errors were removed by visual inspection on the Integrative Genomics Viewer (http://www.broadinstitute.org/igv/) browser. Finally, mutations in 28 driver genes reported in a previous study6 (Supplementary Table 5) and hot spot mutations reported in the COSMIC database with ≥10 mutated tumors, including KRAS and CDKN2A mutations, were considered as driver mutations. To confirm the accuracy of mutation calling, we also called single nucleotide variants using MuTect39 with unmatched control samples with the following parameters: (i) removal of SNPs in ESP, the 1000 genomes project, ExAC and HGVD with minor allele frequency of ≥0.001; (ii) support from ≥5 reads in a tumor; (iii) a VAF ≥ 0.02; (iv) a tumor_alt_fpir_mad > 0. Variants with VAF ≥ 0.4 and synonymous variants were also removed as germline SNPs, except for loss-of-function mutations in tumor suppressor genes and gain-of-function mutations reported in the COSMIC database. We confirmed a high concordance rate between mutations call using both methods, except for a small number of variants with low VAF (Supplementary Fig. 9a).
CN changes were analyzed using CNACS (https://github.com/papaemmelab/toil_cnacs)40, in which the total number of sequencing reads covering each bait region or SNP probe, and the allele frequency of the heterozygous SNP were used as input data. For the identification of regions with LOH, in addition to deletions and copy-neutral LOHs called by CNACS, we identified gains with LOH based on the estimated tumor purities by total and allele specific (As) CN (Supplementary Fig. 9b). For regions with gain (CN = 3), tumor purity was estimated as follows: (by total CN) Purity = Total CN − 2; (by As CN) Purity = 2 × (1 − As CN)/As CN (gain without LOH); and Purity = 2 × (1 − As CN)/(2 + As CN) (gain with LOH).
We used Control-FREEC41 with the contaminationAdjustment option, which corrects for contamination by normal cells, to confirm the LOH status of BRCA1/2 loci determined by CNACS. The median of the total and As CN of probes within BRCA1/2, estimated by CNACS and Control-FREEC, were well correlated (Supplementary Fig. 9c). To further confirm the accuracy of CN calling, we called CN changes using SNP array karyotyping for fresh frozen samples (n = 5). SNP array-based CN analysis was performed using CNAG software42,43. SNP array analysis also provided an almost identical CN profile, including LOH of the BRCA1 and BRCA2 loci (Supplementary Fig. 10), and CNACS detected 57/61 CN alterations identified by SNP array.
Analysis of TCGA dataset
Samples subjected to whole-genome amplification were excluded from analysis to accurately define CN changes and SVs. Sequencing data of 829 WES of breast cancer tumors in the TCGA dataset were downloaded. Variants with a VAF ≥ 0.2 in the germline control sample and with <0.001 minor allele frequency in ESP, the 1000 genomes project, ExAC and HGVD were considered as germline mutations. Tumors with pathogenic germline variants of BRCA1/2 (n = 38) were defined in the same way as our cohort, and those with somatic BRCA1/2 mutations were not included. Four samples were excluded for the following reasons: low quality of CN data (n = 3) and low tumor purity (n = 1). Somatic variants were detected using EBCall with following parameters: (i) VAF in tumor samples ≥0.05; (ii) a P value < 10−1.3 (by Fisher’s test); (iii) P value < 0.0001 (by EBCall). Mutational signatures were analyzed using pmsignature22, and three samples with a high number of artifacts, including TCGA-A2-A0T5, TCGA-A2-A0T6, and TCGA-A7-A0DB were excluded from the analysis. CN changes were also detected from the WES data using CNACS. SVs were analyzed using GenomonSV (https://github.com/Genomon-Project/GenomonSV) as previously reported44 with additional filters, (i) a frequency in the germline sample <0.02; (ii) a P value < 10−1.5 (by Fisher’s test); (iii) a length of overhang ≥ 100. SVs identified in other samples were also removed as germline variants or errors. Clinical information and PAM50 mRNA subtypes of these samples were extracted from the TCGA database.
Statistics and reproducibility
A comparison of categorical variables between mutation carriers and noncarriers was made using the Fisher’s exact test or chi-square test where appropriate. For continuous variables, the Mann–Whitney U test was used for group comparisons. P values less than 0.05 were considered statistically significant. The overall survival time for all patients was determined from the date of diagnosis of breast cancer to the time of last follow-up, or death, by examining medical records. Survival was estimated using the Kaplan–Meier product-limit method and differences were tested for statistical significance using the log-rank test. We performed multivariate regression analysis using the Cox proportional hazards model. All analyses were performed using JMP Pro 14.0.0 software.
No statistical methods were used to determine sample size since this is an exploratory study. We enrolled as many patients as possible who provided consent for our study during the enrollment period between September 2011 and October 2016. A total of 2136 breast cancer patients were enrolled in this study.
Data access
Targeted sequencing data of 106 pooled DNAs and 60 breast cancer samples have been deposited at the European Genome-phenome Archive (https://www.ebi.ac.uk/ega/) under the accession Nos. EGAS00001004630 and EGAS00001004182, respectively.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Easton, D. F. et al. Gene-panel sequencing and the prediction of breast-cancer risk. N. Engl. J. Med. 372, 2243–2257 (2015).
Tung, N. et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J. Clin. Oncol. 34, 1460–1468 (2016).
Sun, J. et al. Germline mutations in cancer susceptibility genes in a large series of unselected breast cancer patients. Clin. Cancer Res. 23, 6113–6119 (2017).
Momozawa, Y. et al. Germline pathogenic variants of 11 breast cancer genes in 7,051 Japanese patients and 11,241 controls. Nat. Commun. 9, 4083 (2018).
Sugano, K. et al. Cross-sectional analysis of germline BRCA1 and BRCA2 mutations in Japanese patients suspected to have hereditary breast/ovarian cancer. Cancer Sci. 99, 1967–1976 (2008).
Nakamura, S. et al. Prevalence and differentiation of hereditary breast and ovarian cancers in Japan. Breast Cancer 22, 462–468 (2015).
Polak, P. et al. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat. Genet. 49, 1476–1486 (2017).
Nik-Zainal, S. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534, 47–54 (2016).
Nielsen, F. C., van Overeem Hansen, T. & Sørensen, C. S. Hereditary breast and ovarian cancer: new genes in confined pathways. Nat. Rev. Cancer 16, 599–612 (2016).
Maxwell, K. N. et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat. Commun. 8, 319 (2017).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Stephens, P. J. et al. The landscape of cancer genes and mutational processes in breast cancer. Nature 486, 400–404 (2012).
Banerji, S. et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 486, 405–409 (2012).
Shah, S. P. et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486, 395–399 (2012).
Nik-Zainal, S. et al. The life history of 21 breast cancers. Cell 149, 994–1007 (2012).
Sanchez-Garcia, F. et al. Integration of genomic data enables selective discovery of breast cancer drivers. Cell 159, 1461–1475 (2014).
Antoniou, A. C. et al. Breast-cancer risk in families with mutations in PALB2. N. Engl. J. Med. 371, 497–506 (2014).
Couch, F. J. et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J. Clin. Oncol. 33, 304–311 (2015).
Sekine, M. et al. Mutational analysis of BRCA1 and BRCA2 and clinicopathologic analysis of ovarian cancer in 82 ovarian cancer families: two common founder mutations of BRCA1 in Japanese population. Clin. Cancer Res. 7, 3144–3150 (2001).
Kim, Y. C. et al. Prevalence and spectrum of BRCA germline variants in mainland Chinese familial breast and ovarian cancer patients. Oncotarget 7, 9600–9612 (2016).
Daly, M., Pilarski, R. & Berry, M. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®Genetic/Familial High-Risk Assessment: Breast and Ovarian Version 1, 2018).
Shiraishi, Y., Tremmel, G., Miyano, S. & Stephens, M. A simple model-based approach to inferring and visualizing cancer mutation signatures. PLoS Genet. 11, e1005657 (2015).
Sorlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100, 8418–8423 (2003).
Foulkes, W. D. et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J. Natl Cancer Inst. 95, 1482–1485 (2003).
Martins, F. C. et al. Evolutionary pathways in BRCA1-associated breast tumors. Cancer Discov. 2, 503–511 (2012).
Skoulidis, F. et al. Germline Brca2 heterozygosity promotes Kras(G12D) -driven carcinogenesis in a murine model of familial pancreatic cancer. Cancer Cell 18, 499–509 (2010).
Konishi, H. et al. Mutation of a single allele of the cancer susceptibility gene BRCA1 leads to genomic instability in human breast epithelial cells. Proc. Natl Acad. Sci. USA 108, 17773–17778 (2011).
Tutt, A. et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat. Med. 24, 628–637 (2018).
Robson, M. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 377, 523–533 (2017).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Drew, Y. et al. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2. J. Natl Cancer Inst. 103, 334–346 (2011).
Jonsson, P. et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature 571, 576–579 (2019).
Bernard, E. et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat. Med. 26, 1549–1556 (2020).
Calvo, S. E. et al. High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat. Genet. 42, 851–858 (2010).
Shiraishi, Y. et al. An empirical Bayesian framework for somatic mutation detection from cancer genome sequencing data. Nucleic Acids Res. 41, e89 (2013).
Yoshida, K. et al. The landscape of somatic mutations in Down syndrome-related myeloid disorders. Nat. Genet. 45, 1293–1299 (2013).
Pritchard, C. C. et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 375, 443–453 (2016).
Zhang, J. et al. Germline mutations in predisposition genes in pediatric cancer. N. Engl. J. Med. 373, 2336–2346 (2015).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213–219 (2013).
Yokoyama, A. et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 565, 312–317 (2019).
Boeva, V. et al. Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics 28, 423–425 (2012).
Nannya, Y. et al. A robust algorithm for copy number detection using high-density oligonucleotide single nucleotide polymorphism genotyping arrays. Cancer Res. 65, 6071–6079 (2005).
Yamamoto, G. et al. Highly sensitive method for genomewide detection of allelic composition in nonpaired, primary tumor specimens by use of affymetrix single-nucleotide-polymorphism genotyping microarrays. Am. J. Hum. Genet. 81, 114–126 (2007).
Kataoka, K. et al. Aberrant PD-L1 expression through 3’-UTR disruption in multiple cancers. Nature 534, 402–406 (2016).
Acknowledgements
We are grateful to the TCGA Research Network (http://cancergenome.nih.gov/) for providing the data analyzed in this manuscript. We would like to thank all of the Kyoto Breast Cancer Research Network (KBCRN) participants, including Mitsuru Tanaka, Susumu Mashima, Tsuyoshi Tachibana, Shingo Sakata, Fumiaki Yotsumoto, Norimichi Kan, and Nobuhiko Shinkura for their help with providing DNA samples and clinical information of the patients in their hospital. We also thank Maki Nakamura for her technical assistance. This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Health, Labor and Welfare of Japan (15H05909) [S.O.]. This research was also partially supported by Astra Zeneca Co., Ltd. We would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Contributions
Y.I.-K., K. Yoshida, N.K.-S., S.O., and M. Toi conceived the project. Y. Shiozawa, H.T., Y. Shiraishi, A.S.-O., Y.N., K.C., and S.M. developed the bioinformatics pipelines of the sequencing data. Y.I.-K. prepared samples and performed the sequencing experiments. Y.I.-K., K. Yoshida, T.N., Y. Shiozawa, Y.T., and Y.I. performed the sequencing data analyses. Y.F. performed SNP array karyotyping. M. Takada and Y. Kataoka helped statistical analyses. M.K., N.S., E.S., M. Takada, M. Torii, H.Y., K.Y., R.O., Y.M., H.K., S.T., A.Y., H.S., and T.I. collected the specimens and clinical information. Y.I.-K., K. Yoshida, S.O., and M. Toi wrote the paper. Y.I.-K. and K. Yoshida generated the figures and tables. S.O. and M. Toi led the entire project. All authors participated in discussions and interpretation of the data and results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing interests: Masakazu Toi: Research grant: Astra Zeneca, Pfizer, Eisai, Chugai Pharma, Astellas Pharma, Daiichi Sankyo, Taiho Pharmaceutical, C & C Research Laboratories, Ono Pharmaceutical, Shimadzu, Nippon-Kayaku, AFI technology, Japan Breast Cancer Research Group. Consulting or Advisory Role: Daiichi Sankyo, Kyowa Hakko Kirin, Athenex Oncology and Bertis. Honoraria as lecture fee: AstraZeneca, Bayer, C & C Research Laboratories, Chugai Pharma, Daiichi Sankyo, Eisai, Eli Lilly, Genomic Health, Konica Minolta, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Sanofi, Shimadzu, Taiho Pharmaceutical, Takeda, Yakult. Travel and accommodation expenses: Genomic Health Inst., Eli Lilly and companies. Others: A member of board of directors (no salary) Organization for Oncology and Translational Research, Japan Breast Cancer Research Group and Kyoto Breast Cancer Research Network.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Inagaki-Kawata, Y., Yoshida, K., Kawaguchi-Sakita, N. et al. Genetic and clinical landscape of breast cancers with germline BRCA1/2 variants. Commun Biol 3, 578 (2020). https://doi.org/10.1038/s42003-020-01301-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-020-01301-9
This article is cited by
-
Environmental impact on carcinogenesis under BRCA1 haploinsufficiency
Genes and Environment (2023)
-
Prevalence of BRCA1 and BRCA2 pathogenic variants in 8627 unselected patients with breast cancer: stratification of age at diagnosis, family history and molecular subtype
Breast Cancer Research and Treatment (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.