Abstract
The recently emerged pachychoroid concept has changed the understanding of age-related macular degeneration (AMD), which is a major cause of blindness; recent studies attributed AMD in part to pachychoroid disease central serous chorioretinopathy (CSC), suggesting the importance of elucidating the CSC pathogenesis. Our large genome-wide association study followed by validation studies in three independent Japanese and European cohorts, consisting of 1546 CSC samples and 13,029 controls, identified two novel CSC susceptibility loci: TNFRSF10A-LOC389641 and near GATA5 (rs13278062, odds ratio = 1.35, P = 1.26 × 10−13; rs6061548, odds ratio = 1.63, P = 5.36 × 10−15). A T allele at TNFRSF10A-LOC389641 rs13278062, a risk allele for CSC, is known to be a risk allele for AMD. This study not only identified new susceptibility genes for CSC, but also improves the understanding of the pathogenesis of AMD.
Similar content being viewed by others
Introduction
Central serous chorioretinopathy (CSC) is a common eye disease characterized by serous retinal detachment of the macular regions and retinal pigment epithelium (RPE)1,2. Although retinal detachments in CSC eyes typically resolve spontaneously, some cases become chronic resulting in permanent retinal tissue damage3,4,5. Additionally, recent studies have shown that the occurrence of choroidal neovascularization (CNV) is a common complication, leading to severe vision loss6,7. Based on these findings, CSC is currently recognized as an important sight-threatening disease that can lead to legal blindness. Although clinical studies have shown that dysfunction of the retinal pigment epithelium and hyperpermeability of the choroidal vessels are essential in the etiology of CSC8, the exact mechanisms underlying CSC pathology remain unknown. Previous studies reported that the risk factors of CSC include glucocorticoid use, increased adrenergic hormones, stress, male gender, Helicobacter pylori infection, and type A personality1,5,9,10,11,12,13,14,15,16.
Recently, the pathological overlap between age-related macular degeneration (AMD), which is a major cause of legal blindness in developed countries, and CSC has received increased attention as they share similar clinical characteristics, including serous retinal detachment, pigment epithelium detachment, and CNV occurrence. Particularly, because CNV secondary to CSC (which was recently named as pachychoroid neovasculopathy because of its characteristic thick choroid17,18,19) often masquerades as AMD20, recent studies highlighted the importance of differentiating AMD and CSC18,19,21,22,23,24,25. Interestingly, although AMD and CSC show similar clinical manifestations, genetic studies revealed that they have contrasting characteristics with respect to complement factor H (CFH), an established AMD susceptibility gene26; the risk alleles for AMD at CFH Y402H and CFH I62V confer a protective effect against CSC27,28,29. Taken together, investigating the genetic background of CSC is currently of great importance and can improve the understanding of the etiology of both CSC and AMD.
Two genome-wide association studies (GWASs) for CSC have been reported30,31. However, both studies have limitations such as a lack of replication studies using independent cohorts and limited sample sizes. Even after considering previous candidate gene studies28,32,33,34, no CSC susceptibility gene other than CFH has been well-replicated and established. Therefore, robust GWASs are needed to further understand the genetic background of CSC.
In the present study, we conducted a large-scale GWAS for CSC followed by replication analyses using three independent Japanese and European cohorts. Our analysis using 1546 CSC cases and 13,029 controls identified two novel CSC susceptible loci, rs13278062 at TNFRSF10A-LOC389641 and rs6061548 near GATA5, which were significantly associated with the disease on a genome-wide scale. As these single-nucleotide polymorphisms (SNPs) showed a robust and homogenous effect among the Japanese and European cohorts, they may be important targets for further molecular biological evaluation and the development of new treatments.
Results
GWAS for CSC and replication analyses in Japanese cohorts
After stringent quality control, 2,893,743 SNPs were included in the first-stage of the GWAS in a Japanese case–control cohort consisting of 610 CSC patients and 2850 controls. We included three principal components as covariates to adjust for possible population stratification, which provided an acceptable control; the genomic inflation factor lambda (λGC) was 1.157. The quantile–quantile plot is shown in Supplementary Fig. 1.
To examine whether a population substructure existed and its influence on the GWAS results, we performed principal component analysis (PCA) for the current study using publicly available multiethnic genotype data from 1000 Genome project (Phase 3, ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/release/20130502/) (Supplementary Fig. 2) and without this data (Supplementary Fig. 3). The analyses revealed that nearly all subjects in our discovery GWAS fell into the Japanese cluster, whereas a mild population substructure existed within the current discovery GWAS.
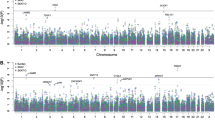
In the first-stage, we identified two loci showing a suggestive P-value of <1.0 × 10−6 for rs13278062 at TNFRSF10A-LOC389641 (ORdiscovery = 1.38, Pdiscovery = 5.94 × 10−7) and rs6061548 near GATA5 (ORdiscovery = 1.61, Pdiscovery = 2.52 × 10−7). The Manhattan plot, regional plots and linkage disequilibrium plots are shown in Figs. 1 and 2 and Supplementary Fig. 4, respectively. Downstream of rs13278062, some SNPs in the CHMP7 region showed relatively low P-values.
Each plot shows −log10-transformed P-values for all SNPs. The horizontal solid line represents the genome-wide significance threshold of P = 5.0 × 10−8, and the lower broken line represents the suggestive threshold of P = 1.0 × 10−6. Two SNPs exceeded the suggestive threshold; rs13278062 at TNFRSF10A-LOC389641 (P = 5.94 × 10−7) and rs6061548 near GATA5 (P = 2.52 × 10−7).
No variants were observed downstream of rs6061548 in the regional plot probably owing to stringent QC. Therefore, we made a regional plot around rs6061548 using the GWAS results before QC was applied. In this regional plot, many SNPs within GATA5 showed low P-values; the most significant association was found at rs13044490 within GATA5 (OR = 1.67, P = 2.94 × 10−10, Supplementary Fig. 5).
In the replication stage using 278 independent CSC samples from Japan, both rs13278062 at TNFRSF10A-LOC389641 (OR = 1.35, P = 8.97 × 10−4) and rs6061548 near GATA5 (OR = 1.39, P = 1.28 × 10−2) showed significant associations with CSC (Table 1).
The associations of two SNPs with CSC were further evaluated in another Japanese CSC case–control dataset. Using the data from Kobe University Hospital, we found that rs6061548 near GATA5 was also significantly associated with CSC in this dataset (OR = 2.29, P = 3.26 × 10−6). Rs13278062 showed a trend towards an association with the same direction of effect (OR = 1.19, P = 0.189, Table 1).
Replication in a European cohort and meta-analysis
We conducted trans-ethnic replication analyses to confirm the association. Association analysis using 521 cases and 3577 controls of European descent revealed that rs13278062 and rs6061548 were significantly associated with CSC in the European cohort (Table 1). The odds ratios of the SNPs in the European cohort were similar to that observed in the Japanese cohort (OREuropean = 1.36, PEuropean = 1.47 × 10−5 for rs13278062, and OREuropean = 1.60, PEuropean = 5.80 × 10−4 for rs6061548). A meta-analysis of the Japanese and European cohorts also revealed strong association between CSC and rs13278062 at TNFRSF10A-LOC389641 (ORmeta-all = 1.35, Pmeta-all = 1.26 × 10−13), as well as rs6061548 near GATA5 (ORmeta-all = 1.63, Pmeta-all = 5.36 × 10−13). As shown in Fig. 3, the effects of rs13278062 and rs6061548 on CSC occurrence were homogenous among the Japanese and European cohorts.
Association of previously reported SNPs with CSC
Previous GWAS of CSC reported two susceptibility loci, CFH rs1329428 and SLC7A5 rs1186504930,31. In the current GWAS, CFH rs1329428 showed a significant association with CSC (OR = 1.17, P = 0.015). SLC7A5 rs11865049 showed the same direction of effect as previously reported but did not reach a significant value (OR = 1.15, P = 0.24). These results are summarized in Table 2.
Expression in human tissue
A search in a publicly available quantitative trait locus analysis (eQTL) database revealed that rs13278062 was significantly associated with TNFRSF10A expression (GTEx Portal. https://gtexportal.org/home/), but not with any other genes nor CHMP7. A multi-tissue eQTL plot revealed that the normalized effect size (NES) of rs13278062 on TNFRSF10A expression was strongest in the adrenal gland (NES = −0.973, P = 4.5 × 10−39; Supplementary Fig. 6). Although it was unclear whether rs6061548 near GATA5 affects the expression or function of any genes in the database, rs13044490 within GATA5, which was a top-hit SNP in the regional plot, was significantly associated with GATA5 expression. The effect of rs13044490 on GATA5 expression was strongest in the esophageal muscularis (NES = 0.347, P = 3.9 × 10−8; Supplementary Fig. 7).
To confirm the expression of the genes in the human retina and choroid that play a role in CSC pathogenesis, we conducted database searching using the Eyeintegration database (https://eyeintegration.nei.nih.gov/, v1.01) and The Ocular Tissue Database (https://genome.uiowa.edu/otdb/), the only databases that includes gene expression data in human retina and choroid. The Eyeintegration database showed that the expression of both TNFRSF10A and GATA5 in the adult human RPE/choroid (n = 48) was stronger than that in the adult human retina (n = 52) as summarized in Fig. 4. These results were supported by The Ocular Tissue Database, which shows that the expression of TNFRSF10A in the adult human RPE/choroid was stronger than that in the adult human retina (PLIER normalized expression level = 18.90 vs. 16.34) and that of GATA5 in the adult human RPE/choroid was also stronger than that in the adult human retina (PLIER normalized expression level = 57.26 vs. 48.30). The Eyeintegration database revealed that the expression of both TNFRSF10A and GATA5 was also observed in other human tissues (Supplementary Note).
Expression levels of TNFRSF10A and GATA5 in the human retina (n = 52) and RPE/choroid (n = 48) are given in transcripts per million (TPM). TNFRSF10A was strongly expressed in the human RPE/choroid compared to in the retina (116.62 ± 58.53 vs. 18.11 ± 8.96 TPM, P < 0.001). GATA5 was also strongly expressed in the human RPE/choroid compared to in the retina (69.05 ± 37.91 vs. 4.66 ± 7.08 TPM, P < 0.001). TPM values are expressed as the mean ± standard deviation and compared by Wilcoxon test. RPE retinal pigment epithelium.
Pathway analysis
We performed pathway analysis using VEGAS2Pathway (https://vegas2.qimrberghofer.edu.au/). In total, 9723 pathways were evaluated. The top ten pathways are shown in Supplementary Table 1. The most significantly associated pathway was the ESCRT-III complex (M00412, P = 2.60 × 10−5). However, no pathways reached the genome-wide, pathway-based significant P-value of <1.0 × 10−530,35.
Discussion
In the current study, we identified two novel susceptible loci, rs13278062 at TNFRSF10A-LOC389641 and rs6061548 near GATA5 for a common pachychoroid disease, CSC, through a large GWAS followed by replication studies in three independent case–control datasets of Japanese and European origin. These SNPs showed robust and consistent association among all datasets. Interestingly, rs13278062 has been reported to be a susceptibility SNP for AMD. As the relationship between AMD and CSC has received increased attention, the current results have important scientific implications that improve the understanding of the similarities and differences between AMD and CSC.
TNFRSF10A was first identified as an AMD susceptibility locus in a Japanese population36. Although this association has been confirmed in other ethnicities, the effect of TNFRSF10A on AMD in Caucasians was reported to be weaker than that in Asian population37,38,39. Recently, some researchers reported a subgroup within AMD that incorporates the characteristics of CSC, such as thick choroid and choroidal vascular hyperpermeability7,17,19. Although the precise rate of the subgroup among patients with AMD is currently unknown, the rate is estimated to be higher in Asians than in Caucasians22,40. Taken together with the current result, we speculate that eyes with CNV, which is traditionally diagnosed as AMD, include CNV secondary to CSC, which may occur more frequently in Asians compared to that Caucasians. In support of this, the effect of TNFRSF10A on CSC occurrence (OR = 1.38) in the present study was higher than that of traditional AMD occurrence in a previous study (ORforAMD = 1.25 in Asian, and ORforAMD = 1.11 in European)39. It is also possible that TNFRSF10A has pleiotropic effects on both AMD and CSC. The effects of TNFRSF10A on AMD should be further evaluated while stratifying the data for the presence of a pachychoroid background.
CNV accompanied by the characteristics of CSC was recently termed as pachychoroid neovasculopathy, as the thickened choroid is the most characteristic clinical feature of the condition. Gene expression evaluation in ocular tissue supports the importance of the RPE/choroid regarding the etiology of pachychoroid neovasculopathy and CSC, as TNFRSF10A is more strongly expressed in the RPE/choroid than in the retina. However, the exact role of TNFRSF10A in CSC occurrence or choroidal structure is unclear. Considering that the adrenergic hormones are established risk factors for CSC41,42, and that the eQTL showed that the genotype of rs13278062 at TNFRSF10A was strongly associated with its expression in the adrenal gland, TNFRSF10A may affect the risk of CSC by modulating hormone secretion from the adrenal glands.
We additionally identified that rs6061548 near GATA5 was associated with CSC. GATA5 is known to play an important role in vascular system development43,44,45. A recent study showed that GATA5 is expressed in the microvascular endothelial cells and that its inactivation in mice results in vascular endothelial dysfunction46. Considering the stronger expression of GATA5 in the RPE/choroid than in the retina, we speculate that GATA5 may affects the susceptibility to CSC through vascular endothelial dysfunction in the choriocapillaris, which constitutes the inner vascular layers of the choroid, composed largely of fenestrated capillaries. This hypothesis is compatible with previous reports showing that eyes with CSC had choriocapillary hypoperfusion47,48, and primary choroidal ischemia48. GATA5 is also known to play an important role in stomach development and gastric diseases49,50,51. Interestingly, GATA5 is reported to be upregulated by H. pylori infection52, which is one of the risk factors of CSC10,12,13,14. As described above, GATA5 may also be an important target for the further understandings of CSC.
The current study has many strengths and limitations. The main strengths are its large sample size and that multiple replication studies were performed in different ethnic groups, which led to robust results. As CSC is thought to be a benign, self-limiting disease, its genetic background has not been clarified. However, the pathology of CSC has become an important issue in relation to AMD. To our knowledge, this study is the largest genetic study on CSC and the first study to identify transethnically robust susceptible genes using a non-hypothesis-driven, exploratory approach. Considering the identification of relatively high, consistent effects across multiple ethnic groups, the current study has strong scientific implications. However, the sample size of this study was still limited. An even larger sample size is required to identify additional susceptibility SNPs with low allele frequency or low effect size and to further elucidate disease pathways in CSC. Another limitation is the inflated λGC in our discovery GWAS, which may have led to false-positive associations. This inflation may be related to the presence of a mild population substructure, as inflation was still observed even after conducting the GWAS using samples genotyped with a single-DNA microarray platform, OmniExpress (λGC = 1.098). However, the positive associations of both TNFRSF10A-LOC389641 rs13278062 and near GATA5 rs6061548 were still significant even after genomic control correction (Pmeta = 2.57 × 10−12 and Pmeta = 6.29 × 10−12, respectively; Supplementary Table 2), and thus these associations appear to be robust.
In summary, we identified two novel CSC susceptibility loci, rs13278062 at TNFRSF10A-LOC389641 and rs6061548 near GATA5, using a total of 1546 CSC cases and 13,029 controls. These variants showed robust, consistent, and mild to moderate associations with CSC across ethnicities. We confirmed that both genes are expressed in the choroid, which is the main focus of CSC and AMD. Our findings improve the understanding of the pathogenesis of CSC and AMD.
Methods
Patient enrollment
In the discovery GWAS, 610 Japanese patients with CSC were recruited from the Kyoto University Hospital, and 2850 healthy Japanese controls were recruited from the Aichi Cancer Center Research Institute, Hayashi Eye Hospital, Mizoguchi Eye Hospital, Oita University Faculty of Medicine, Ideta Eye Hospital, Shinjo Eye Clinic, Miyata Eye Hospital, Ozaki Eye Hospital, Kyoto University Hospital, and Nagahama City Hospital. Detailed information of the control cohort is described elsewhere53, and is briefly summarized in the Supplementary Note and Supplementary Table 3.
In the first replication stage, 278 CSC patients were recruited from across Japan, particularly Kyoto University Hospital (N = 57), Kagawa University Hospital (N = 104), Yamanashi University Hospital (N = 80), and Fukushima Medical University Hospital (N = 37). Control allele frequency data were extracted from the genome-wide dataset of healthy Japanese subjects from the Tohoku Medical Megabank Organization (N = 3498)54,55,56, Kyoto University (N = 3074)57,58, and Yokohama City University dataset (N = 1048). Detailed information is shown in the Supplementary Note. In the second and the third replication stages, the Kobe CSC case–control dataset, which consists of 137 Japanese patients with CSC and 1153 Japanese controls, and European CSC case–control dataset, which consists of 521 European patients with CSC and 3577 European controls, were utilized, respectively. Although the detailed information for this dataset has been described previously30,31, a brief summary is also provided in the Supplementary Note.
All procedures adhered to the tenets of the Declaration of Helsinki. The Institutional Review Board and Ethics Committee of each participating institute approved the respective study protocols. All patients were fully informed of the purpose and procedures of the study, and written consent was obtained from all patients prior to their participation in the study.
Diagnosis of patients with CSC
All patients underwent a comprehensive ophthalmic examination, including visual acuity measurement; slit-lamp biomicroscopy; dilated fundoscopy; color fundus photography; and optical coherence tomography (OCT) examination, including enhanced depth imaging, fundus autofluorescence, fluorescein angiography, and indocyanine green angiography CSC was diagnosed based on medical history, serous retinal detachment, thickened choroid with dilated choroidal vessels seen on OCT, choroidal vascular hyperpermeability on ndocyanine green angiography, and/or leakages on fluorescein angiography at the level of the RPE. Based on these findings, two retina specialists (M.M. and Y.H.) diagnosed the patients independently, and discrepancies were adjusted in a face-to-face consensus session. Patients with other causes of fluorescein leakage or serous retinal detachment unrelated to CSC (e.g., AMD, intraocular inflammation, diabetic retinopathy, or retinal vein occlusion) were excluded from the study. In the second and third replication stages, patients with CSC were diagnosed based on previously described criteria29,30,31. The details are summarized in the Supplementary Note.
Genotyping
Genomic DNA was extracted from peripheral blood samples according to standard laboratory procedures. A series of BeadChip DNA arrays (Illumina, San Diego, CA, USA), namely OmniExpress (N = 250) and Asian Screening Array (N = 360), were used for genotyping the CSC samples, while Human610-Quad BeatChip (N = 1194) and OmniExpress (N = 1656) were used for genotyping the control samples.
Genotype imputation was performed using the Michigan imputation server (https://imputationserver.sph.umich.edu/index.html#!pages/home) with the 1000 Genome dataset (phase3 v5 release) of East Asians as a reference panel for each dataset. In each dataset, SNPs with a call rate <90% or a minor allele frequency <1% were excluded before genotype imputation. Imputed SNPs for which R2 was <0.9 were excluded from the subsequent association analysis. Next, SNPs with a call rate <90%, a minor allele frequency <1%, or significant deviation (P < 1.0 × 10−5) from Hardy–Weinberg equilibrium were excluded from further statistical analysis, and samples with a call rate <90% were also excluded. We checked the allelic discrimination of SNPs showing a suggestive association with CSC in the discovery GWAS (P < 1.0 × 10−5) for each platform. We excluded SNPs with an insufficient quality of allelic discrimination and their proxy SNPs (R2 > 0.8). Finally, 2,893,743 SNPs from 610 CSC samples and 2850 control samples were used for discovery stage analysis.
In the first replication stage, genotypes of CSC samples (N = 278) were determined using a commercially available assay (TaqMan SNP assay with the ABI PRISM 7700 system; Applied Biosystems, Foster City, CA, USA). We extracted the allele frequency from existing healthy Japanese genome-wide datasets, which included the Tohoku dataset54,55,56, Kyoto University57,58, and Yokohama City University datasets. The details of the genotyping methods are summarized in the Supplementary Note. Briefly, the Integrative Japanese Genome Variation Database (ver 3.5 K JPN, https://ijgvd.megabank.tohoku.ac.jp/) provides genomic reference panels obtained from 3554 normal Japanese subjects recruited from the Tohoku Medical Megabank Organization, Iwate Medical Megabank Organization, Nagahama Prospective Cohort for Comprehensive Human Bioscience, and National Hospital Organization Nagasaki Medical Center. All DNA samples were whole-genome-sequenced using the Illumina HiSeq 2500. The Human Genetic Variation Database is a database of genomic reference panels released from Kyoto University (http://www.hgvd.genome.med.kyoto-u.ac.jp/index.html). This database is a web-accessible resource of genetic variations in the Japanese population. Whole-genome SNV genotyping was performed for 3712 individuals, who formed a subset of participants of The Nagahama Prospective Genome Cohort for the Comprehensive Human Bioscience (the Nagahama Study), with the Illumina HumanHap610 quad, Omni 2.5 M and Human exome Beadarrays (Illumina). The Yokohama City University dataset includes 1048 Japanese healthy controls recruited from the Yokohama City University, Okada Eye Clinic, and Aoto Eye Clinic in Yokohama, Kanagawa Prefecture, Japan. Genotypes of samples from Yokohama City University were determined using BeadChip DNA arrays, namely Human OmniExpress chip (Illumina), with the standard protocol recommended by each manufacturer.
The Kobe CSC case–control dataset, which was used in the second replication stage, was genotyped using the Human OmniExpress BeadChips (Illumina, San Diego, CA, USA) as described elsewhere31. Genomic imputation was performed for the dataset using the BEAGLE 4.1 and 1000 genomes dataset (phase3 v5 release) as the reference panels. Imputed SNPs for which R2 < 0.7 were excluded from the imputed dataset. The association of SNPs with CSC was tested by logistic regression analysis with no adjustment. The European CSC case–control dataset, which was used in the third replication stage, was genotyped using OmniExpress-12 or OmniExpress-24chip. The data were imputed with the Haplotype Reference Consortium release 1.1.2016, and were stringent quality controls were performed as described previously30.
Statistical analyses
Genome-wide logistic regression analysis was conducted for the CSC, adjusting for three principal components. As on age and sex was not available for 1656 out of the 2850 control samples, adjustment for these factors was not performed in the discovery GWAS. Experiment-wide significance in the discovery stage was set to P = 5.0 × 10−8. We carried SNPs with P-value of <1.0 × 10−6 forward to the replication stage. In the first and second replication stages, logistic regression was performed for the SNPs that showed suggestive association in the first-stage. All meta-analyses were performed using the fixed-effect model. Thereafter, differences were considered significant at P < 0.05. Deviations in genotype distributions from Hardy–Weinberg equilibrium were assessed with chi-square tests. These statistical analyses were performed with R ver 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria; available at http://www.rproject.org/), GCTA ver. 1.25.3 (http://cnsgenomics.com/software/gcta/index.html#Overview) and PLINK ver. 2.0 (https://www.cog-genomics.org/plink/2.0/).
In the third replication stage using the European dataset, association analysis was performed using the Firth bias-corrected likelihood ratio test, implemented in EPACTS (version 3.2.6, https://genome.sph.umich.edu/wiki/EPACTS; University of Michigan), correcting for sex and the first two components of ancestry analysis.
Pathway analysis
Using the GWAS summary statistics and P-values of replicated SNPs, we performed gene-based analysis and clustered genes into pathways using VEGAS2pathway (https://vegas2.qimrberghofer.edu.au/, version 2). Briefly, VEGAS2 successively prunes the list of variants with R2 criteria of 0.99, 0.90, 0.70, and 0.50, if a gene contains >1000 SNPs. After each pruning interval, VEGAS2 checks the number of pruned SNPs. If the number of pruned SNPs is <1000, VEGAS2 uses the pruned SNPs from that interval to perform gene-based analysis; otherwise, it iteratively applies an increasingly stringent R2 criteria on all SNPs in the gene. After applying a pruning criterion of R2 = 0.50, it uses all pruned SNPs for analysis irrespective of the number. The gene list was obtained from the VEGAS2 official site (https://vegas2.qimrberghofer.edu.au/glist-hg19). Obtained gene-based result was used to perform calculate pathway-based tests and empirical P-values to obtain the significance of each pathway. Regions +/− 0 kb outside of genes were defined as gene regions, and all SNPs were used for analysis. For the SNPs that were carried forward for to the replication stage, P-values from meta-analysis were used rather than GWAS P-values. The Biosystems gene-pathway annotation file was obtained from the VEGAS2 official site (https://vegas2.qimrberghofer.edu.au/biosystems20160324.vegas2pathSYM). The significance threshold of the empirical P-value in the pathway analysis was set to 1.0 × 10−530,35.
Database search
To determine the existence of a population substructure and its influence on the GWAS results, publicly available genotype data from five populations, including African, South Asian, European, East Asian, and Japanese (1000 Genome project, Phase 3, ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/release/20130502/) were used for PCA.
Searching of the publicly available quantitative trait locus analysis (eQTL) database (Genotype-Tissue Expression (GTEx) Portal: https://gtexportal.org/home/) revealed an association between the SNP genotypes and gene expression in multiple human tissues. NES is defined as the slope of the linear regression and is computed as the effect of the alternative allele relative to the reference allele.
Expression of genes in the adult human retina and RPE was explored in the eyeintegration database (https://eyeintegration.nei.nih.gov/, v1.01. accessed 10 April 2019). The database lists the expression levels of genes given in transcripts per million. Gene expression levels were compared by Wilcoxon test. Expression of genes in the human retina and choroid was explored in The OCULAR TISSUE DATABASE (https://genome.uiowa.edu/otdb/, accessed 28 February 2019). This database provides quantitative expression level of genes in ocular tissues determined by the PLIER (Probe Logarithmic Intensity Error) algorithm.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Top SNPs (n = 100) in the discovery GWAS can be available as Supplementary Data 1. The source data about the expression of genes in the adult human retina and RPE can be available as Supplementary Data 2. The complete GWAS summary data can be visualized here: https://figshare.com/articles/CSC_control_PC3_assoc_logistic/11136047. The datasets generated during the current study are also available from the corresponding author on reasonable request.
References
Gemenetzi, M., De Salvo, G. & Lotery, A. J. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye 24, 1743–1756 (2010).
Kitzmann, A. S., Pulido, J. S., Diehl, N. N., Hodge, D. O. & Burke, J. P. The incidence of central serous chorioretinopathy in Olmsted county, Minnesota, 1980-2002. Ophthalmology 115, 169–173 (2008).
Nicholson, B., Noble, J., Forooghian, F. & Meyerle, C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv. Ophthalmol. 58, 103–126 (2013).
Bujarborua, D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol. Scand. 79, 417–421 (2001).
Breukink, M. B. et al. Chronic central serous chorioretinopathy: long-term follow-up and vision-related quality of life. Clin. Ophthalmol. 11, 39–46 (2016).
Mrejen, S. et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology https://doi.org/10.1016/j.ophtha.2018.12.048 (2019).
Shiragami, C. et al. Clinical features of central serous chorioretinopathy with Type 1 choroidal neovascularization. Am. J. Ophthalmol. 193, 80–86 (2018).
Daruich, A. et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 48, 82–118 (2015).
Yannuzzi, L. Type-A behavior and central serous chorioretinopathy. Retina 7:2, 111–131 (1987).
Haimovici, R., Koh, S., Gagnon, D. R., Lehrfeld, T. & Wellik, S. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology 111, 244–249 (2004).
Weenink, A. A. C., Borsje, R. A. & Oosterhuis, J. A. J. A. Familial chronic central serous chorioretinopathy. Ophthalmologica 215, 183–187 (2001).
Mateo-Montoya, A. & Mauget-Faÿse, M. Helicobacter pylori as a risk factor for central serous chorioretinopathy: literature review. World J. Gastrointest. Pathophysiol. 5, 355–358 (2014).
Bagheri, M., Rashe, Z., Ahoor, M. H. & Somi, M. H. Prevalence of Helicobacter pylori infection in patients with central serous chorioretinopathy: a review. Med. hypothesis, Discov. Innov. Ophthalmol. J. 6, 118–124 (2017).
Zavoloka, O. Helicobacter pylori is the culprit behind central serous chorioretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 254, 2071 (2016).
Wang, M. et al. Central serous chorioretinopathy. Acta Ophthalmol. 86, 126–145 (2008).
Spaide, R. F. et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology 103, 2070–2080 (1996).
Pang, C. E. & Freund, K. B. Pachychoroid neovasculopathy. Retina 35, 1–9 (2015).
Lehmann, M., Bousquet, E., Beydoun, T. & Behar-Cohen, F. Pachychoroid: an inherited condition? Retina 35, 10–16 (2015).
Miyake, M. et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci. Rep. 5, 16204 (2015).
Fung, A. T., Yannuzzi, L. A. & Bailey Freund, K. Type 1 (Sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 32, 1829–1837 (2012).
Azar, G. et al. Pachychoroid neovasculopathy: aspect on optical coherence tomography angiography. Acta Ophthalmol. 95, 421–427 (2017).
Cheung, C. M. G. et al. Pachychoroid disease. Eye 33, 14–33 (2019).
Gallego-Pinazo, R., Dolz-Marco, R. & Freund, K. B. in Choroidal Disorders. 161–170 (Academic Press, 2017).
Hata, M. et al. Intraocular vascular endothelial growth factor levels in pachychoroid neovasculopathy and neovascular age- related macular degeneration. Investig. Ophthalmol. Vis. Sci. 58, 292–298 (2017).
Akkaya, S. Spectrum of pachychoroid diseases. Int. Ophthalmol. 1–8, https://doi.org/10.1007/s10792-017-0666-4 (2017).
Klein, R. J., Robert, J., Emily, Y. & Tsai, J. Complement factor H polymorphism in age-related macular degeneration. Sci. (80−.). 308, 385–389 (2005).
Miki, A. et al. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology 121, 1067–1072 (2014).
Hosoda, Y. et al. CFH and VIPR2 as susceptibility loci in choroidal thickness and pachychoroid disease central serous chorioretinopathy. Proc. Natl Acad. Sci. USA 115, 6261–6266 (2018).
De Jong, E. K. et al. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology 122, 562–570 (2015).
Schellevis, R. L. et al. Role of the complement system in chronic central serous chorioretinopathy: a genome-wide association study. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2018.3190 (2018).
Miki, A. et al. Genome-wide association study to identify a new susceptibility locus for central serous chorioretinopathy in the Japanese population. Investig. Opthalmol. Vis. Sci. 59, 5542 (2018).
Dijk, E. et al. Association of a haplotype in the NR3C2 gene, encoding the mineralocorticoid receptor, with chronic central serous chorioretinopathy. Ophthalmology 135, 446–451 (2017).
Mohabati, D. et al. Genetic risk factors in acute central serous chorioretinopathy. Retina 1, https://doi.org/10.1097/IAE.0000000000002333 (2018).
Breukink, M. B. et al. Genomic copy number variations of the complement component C4B Ggene are associated with chronic central serous chorioretinopathy. Invest. Ophthalmol. Vis. Sci. 56, 5608–5613 (2015).
Mishra, A. & MacGregor, S. A novel approach for pathway analysis of GWAS data highlights role of BMP signaling and muscle cell differentiation in colorectal cancer susceptibility. Twin Res. Hum. Genet. 20, 1–9 (2017).
Arakawa, S. et al. Genome-wide association study identifies two susceptibility loci for exudative age-related macular degeneration in the Japanese population. Nat. Genet. 43, 1001–1005 (2011).
Fritsche, L. G. et al. Seven new loci associated with age-related macular degeneration. Nat. Genet. 45, 433–439 (2013).
Sun, Y. et al. TNFRSF10A-LOC389641 rs13278062 but not REST-C4orf14-POLR2B-IGFBP7 rs1713985 was found associated with age-related macular degeneration in a Chinese population. Invest. Ophthalmol. Vis. Sci. 54, 8199–8203 (2013).
Fritsche, L. G. et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 48, 134–143 (2016).
Cheung, C. M. G. et al. Association between choroidal thickness and drusen subtypes in age-related macular degeneration. Ophthalmol. Retin. 2, 1196–1205 (2018).
Gass, J. D. M. & Little, H. Bilateral bullous exudative retinal detachment complicating idiopathic central serous chorioretinopathy during systemic corticosteroid therapy. Ophthalmology 102, 737–747 (1995).
Bouzas, E. A., Karadimas, P. & Pournaras, C. J. Central serous chorioretinopathy and glucocorticoids. Surv. Ophthalmol. 47, 431–448 (2002).
Jiang, J. -Q. et al. Prevalence and spectrum of GATA5 mutations associated with congenital heart disease. Int. J. Cardiol. 165, 570–573 (2013).
Stennard, F. A. et al. Cardiac T-box factor Tbx20 directly interacts with Nkx2-5, GATA4, and GATA5 in regulation of gene expression in the developing heart. Dev. Biol. 262, 206–224 (2003).
Reiter, J. F. et al. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 13, 2983–2995 (1999).
Messaoudi, S. et al. Endothelial Gata5 transcription factor regulates blood pressure. Nat. Commun. 6, 8835 (2015).
Cakir, B. et al. OCT Angiography of the choriocapillaris in central serous chorioretinopathy: a quantitative subgroup analysis. Ophthalmol. Ther. https://doi.org/10.1007/s40123-018-0159-1 (2019).
Rochepeau, C. et al. Optical coherence tomography angiography quantitative assessment of choriocapillaris blood flow in central serous chorioretinopathy. Am. J. Ophthalmol. 194, 26–34 (2018).
Wang, X. et al. Epigenetic subgroups of esophageal and gastric adenocarcinoma with differential GATA5 DNA methylation associated with clinical and lifestyle factors. PLoS ONE https://doi.org/10.1371/journal.pone.0025985 (2011).
Sobota, R. S. et al. Epigenetic and genetic variation in GATA5 is associated with gastric disease risk. Hum. Genet. 8, 895–906 (2016).
Fukuda, K. & Yasugi, S. The molecular mechanisms of stomach development in vertebrates. Dev. Growth Differ. 6, 375–382 (2005).
Wen, X. Z. et al. Methylation of GATA-4 and GATA-5 and development of sporadic gastric carcinomas. World J. Gastroenterol. 16, 1201–1208 (2010).
Aung, T. et al. A common variant mapping to CACNA1A is associated with susceptibility to exfoliation syndrome. Nat. Genet. 47(Apr), 387–392 (2015).
Kuriyama, S. et al. The Tohoku medical megabank project: design and mission. J. Epidemiol. 26, 493–511 (2016).
Yamaguchi-Kabata, Y. et al. iJGVD: an integrative Japanese genome variation database based on whole-genome sequencing. Hum. Genome Var. 2, 15050 (2015).
Nagasaki, M. et al. Rare variant discovery by deep whole-genome sequencing of 1,070 Japanese individuals. Nat. Commun. 6, 8018 (2015).
Narahara, M. et al. Large-scale East-Asian eQTL mapping reveals novel candidate genes for LD mapping and the genomic landscape of transcriptional effects of sequence variants. PLoS ONE 9, e100924 (2014).
Higasa, K. et al. Human genetic variation database, a reference database of genetic variations in the Japanese population. J. Hum. Genet. 61, 547–553 (2016).
Acknowledgements
We thank Ms. Hatsue Hamanaka for assistance with genotyping. We thank for Dr. Masato Akiyama for supervising about method of the pathway analysis.
Author information
Authors and Affiliations
Contributions
Y.H., M.M., R.Y., F.M., K.Y., and A.T. designed the study; Y.H., M.M., R.L.S., C.J.F.B., C.B.H., A.Mi., A.Me., Y.S., S.Y., Y.T., M.H., Y.M., H.N., A.O., S.O., H.T., A.U., M.M., A.Tak., N.U.A., A.Taj., T.S., N.M., C.S., T.I., S.H., E.K.D.J., A.I.D.H., K.Y., and A.T. performed the study; Y.H., M.M., R.L.S., C.J.F.B., C.B.H., A.Mi., A.Me., A.Tak., T.S., N.M., C.C.K., T.Y.W., R.Y., S.H., E.K.D.J., A.I.D.H., K.Y., and A.Taj. analyzed the data; and Y.H., M.M., and K.Y. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hosoda, Y., Miyake, M., Schellevis, R.L. et al. Genome-wide association analyses identify two susceptibility loci for pachychoroid disease central serous chorioretinopathy. Commun Biol 2, 468 (2019). https://doi.org/10.1038/s42003-019-0712-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-019-0712-z
This article is cited by
-
Contribution of common and rare variants to Asian neovascular age-related macular degeneration subtypes
Nature Communications (2023)
-
Distinct characteristics of central serous chorioretinopathy according to gender
Scientific Reports (2022)
-
Pathophysiology of central serous chorioretinopathy: a literature review with quality assessment
Eye (2022)
-
Association between central serous chorioretinopathy susceptibility genes and choroidal parameters
Japanese Journal of Ophthalmology (2022)
-
Validation study of the claims-based definition for age-related macular degeneration at a single university hospital in Japan
Japanese Journal of Ophthalmology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.