Abstract
In mammals, blood glucose levels likely play a role in appetite regulation yet the mechanisms underlying this phenomenon remain opaque. Mechanisms can often be explored from Drosophila genetic approaches. To determine if circulating sugars might be involved in Drosophila feeding behaviors, we scored hemolymph glucose and trehalose, and food ingestion in larvae subjected to various diets, genetic mutations, or RNAi. We found that larvae with glucose elevations, hyperglycemia, have an aversion to feeding; however, trehalose levels do not track with feeding behavior. We further discovered that insulins and SLC5A11 may participate in glucose-regulated feeding. To see if food aversion might be an appropriate screening method for hyperglycemia candidates, we developed a food aversion screen to score larvae with abnormal feeding for glucose. We found that many feeding defective larvae have glucose elevations. These findings highlight intriguing roles for glucose in fly biology as a potential cue and regulator of appetite.
Similar content being viewed by others
Introduction
In the past few decades, there has been a steady rise in the worldwide incidence of type 2 diabetes. This increase has been linked to greater energy intake relative to output, due to increased food consumption, and subsequent weight gain and obesity1,2,3,4,5,6. In mammals, food consumption is regulated by a variety of inputs, one of which appears to be blood glucose levels7,8. In 1955, Jean Mayer proposed a glucostatic theory in which transient decreases and increases in blood glucose concentrations perceived by specialized “glucoreceptors” on hypothalamic cells trigger perceptions of hunger and satiety, respectively7,8. A more recent study observed that small declines in blood glucose levels often preceded spontaneous meal initiation in rats9. Thus, at least in principle, depletion of fuel substrates should trigger initiation of feeding while repletion should cause cessation of feeding7,10. This notion is also well illustrated in people with insulin secreting tumors11.
The regulation of appetite and glycemia is likely the outcome of a complex interplay of multiple metabolic, hormonal, and neural signals that are not yet completely elucidated10,12,13. Studies in mice and rats have uncovered numerous anorexigenic “satiety factors”, for example, cholecystokinin, glucagon, and leptin, while orexigenic factors, such as ghrelin, stimulate food intake via regulation of the Neuropeptide Y axis10,14,15. As in mammals, neural signals have been implicated in the control of fly feeding behaviors. For example, activation of insulin signaling in the nervous system suppresses larval feeding and overexpression of Neuropeptide F, the fly homolog of mammalian neuropeptide Y, promotes feeding, even of noxious or unfamiliar foods16,17,18,19. Other fly neuropeptides linked to the regulation of food intake include short neuropeptide F and CCHAmide-2, and drosulfakinin and hugin, which increase or decrease larval feeding, respectively20,21,22,23.
To attempt to explore a potential relationship between circulating carbohydrates and feeding behavior, we probed mid-third instar larvae, as their incessant feeding at a consistent rate provides a potentially stable state. Feeding larvae maintain glucose levels between 6–9 mg/dl and trehalose levels at ≥ 1500 mg/dL24, hinting at a highly efficient carbohydrate regulatory mechanistic. We found that increasing the concentration of dietary sucrose was associated with increased levels of circulating glucose and trehalose but decreased rates of feeding in larvae. Further, we observed that feeding aversion appeared to correlate with increasing glucose, but not trehalose, levels. Moreover, severe hyperglycemia also delayed larval foraging activity, indicative of enhanced satiety. Alleviation of hyperglycemia by fasting or through pharmacological means rescued the feeding defect in “flyabetic” larvae. Further, insulin signaling was increased in food averse hyperglycemic larvae. SLC5A11, a sodium/solute co-transporter-like neuronal protein showed decreased expression, and appears to be regulated by insulin activity. Microarray analyses of flyabetic larvae further validated the roles of insulin signaling and SLC5A11 in glucose sensing, and identified other potential candidates. Finally, we evaluated feeding behavior of >2000 P-element lines and identified 63 ‘satiety’ mutants that displayed a very marked aversion to food; ~50% of these were hyperglycemic, supporting the notion that food-averse mutants are an enriched pool to identify genes that regulate glucose levels. Taken together, our data support the notion that there is a strong correlation between hemolymph glucose levels and Drosophila feeding behavior, and that glucose may act as a cue to modulate feeding behavior.
Results
Hemolymph carbohydrates may modulate larval feeding behavior
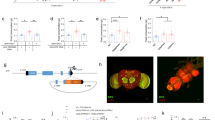
We previously noticed during RNAi screening for glucose regulatory genes24 that hyperglycemic larvae appeared to have a delayed interest in feeding when transferred to fresh media. To attempt to investigate a potential connection between circulating carbohydrates and food consumption, we reared (“chronic conditioning”) mid-third instar w1118 larvae on 1%, 5% (standard food), and 20% sugar media. We then measured the levels of glucose and trehalose in w1118 larval hemolymph (Fig. 1a), and found that, relative to larvae fed 5% sugars, the 1% sugar-fed larvae had slightly reduced glucose (0.8 fold), but not trehalose, levels, and that 20% sugar feeding produced both hyperglycemia (3.3 fold) and hypertrehalosemia (1.4 fold) (Fig. 1b). We then transferred larvae from the 1%, 5%, and 20% sugar media to 1 h of post-conditioning culture in a 20% sucrose solution supplemented with Coomassie dye, to allow us to visualize and to quantitate food consumption (Fig. 1a). The larvae reared on 1% and 5% sugar media had roughly equal gut dye intensity in the visual assay (Fig. 1c). However, the 20% sugar-fed cohorts had visibly less food in their gut after 1 h of post-conditioning dye feeding (Fig. 1c). To more precisely measure food consumption, we extracted Coomassie from larvae and quantitated dye levels using a spectrometric assay, which showed that 1% sugar-reared w1118 larvae ingested 32% more sucrose, while the 20% sugar-reared larvae reduced sucrose intake by 65%, relative to 5% sugar-reared euglycemic counterparts (Fig. 1d). These data indicate a potential link between hemolymph carbohydrates and feeding. Further, the observations that the 1% cohorts displayed increased food intake and low glucose levels, but normal trehalose levels hint at the possibility that hemolymph glucose, rather than trehalose, might be the carbohydrate link to feeding behavior control.
Changes in hemolymph carbohydrates appears to influence sucrose ingestion by larvae. a Larvae chronically reared on media containing different concentrations of sugars were subjected to glucose, trehalose, and post-conditioning feeding assays. b Circulating glucose (left) and trehalose (right) levels were measured in mid-third instar w1118 larvae cultured in media containing 1%, 5% or 20% sugars. n ≥ 4 each (≥10 larvae per replicate). c, d Relative dye ingested in 1 h of post-conditioning 20% sucrose feeding was observed (c) and measured (d) in w1118 larvae cultured on 1%, 5%, or 20% sugars. ≥ 50 larvae per treatment. For dye quantification, n ≥ 3 (20 larvae per replicate). Error bars indicate standard deviation (s.d.). Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001
We then measured feeding in another setting where glucose, but not trehalose, levels are changed: larvae subjected to acute feeding of concentrated sucrose respond by increasing hemolymph glucose levels, but they do not alter trehalose levels24,25. Therefore, we transferred w1118 larvae reared on standard food to 4 h of “acute” conditioning in water, 5% sucrose, 20% sucrose, or 2.5% Splenda i.e. sucralose (equivalent sweetness to 20% sucrose, negligible calories) solutions (without dye). After 4 h, half of the larvae from each of the four groups were analyzed for post-conditioning carbohydrate levels, while the others were transferred to a 1 h incubation in Coomassie dye-supplemented 20% sucrose solution to assess post-conditioning food intake (Fig. 2a). All the larvae had equal trehalose levels, while the larvae acutely conditioned on 20% sucrose for 4 h had significantly increased glucose levels by 3.6-fold (Fig. 2b). These hyperglycemic larvae also had significantly reduced food ingestion by 22%, relative to the euglycemic larvae conditioned on water, Splenda, or 5% sucrose (Fig. 2c), supporting a potential association between hyperglycemia and decreased feeding in high sucrose-conditioned w1118 larvae. The larvae fed 2.5% Splenda ate slightly more (1.8-fold) of the post-conditioning media despite no significant decrease in glycemia (Fig. 2b, c). It appears that larvae may simply prefer the taste of Splenda, as w1118 larvae consumed the same amount of water, 5% sucrose, and 20% sucrose but ingested increased amounts (1.6 fold) of 2.5% Splenda in the first hour of conditioning (Supplementary Fig. 1b). Taken together, these data support the notion that circulating glucose, but not trehalose, levels appear to be one of the regulators of larval feeding behavior.
Glucose levels but not trehalose levels may be linked to altered feeding by larvae. a w1118 controls reared in standard media were provided water, 2.5% Splenda, 5% sucrose, or 20% sucrose and after 4 h of acute conditioning carbohydrate levels and dye ingested in 1 h of post-conditioning feeding were measured. b Relative glucose (left) and trehalose (right) levels were measured in w1118 larvae after 4 h conditioning on different media. n ≥ 4 each (≥10 larvae per replicate). c Quantification of post-conditioning feeding (1 h) in larvae. n ≥ 8 each (20 larvae per replicate). Error bars indicate s.d. Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001
Elevations in glucose correlate with reduced feeding
To attempt to further discriminate whether the glucose and/or trehalose fraction might regulate larval feeding, we probed collections of P-element metabolic mutants24. For these larger-scale efforts, we developed a qualitative dual-dye visual liquid feeding assay amenable to high throughput analyses (Fig. 3a). In this assay, we cultured control and mutant larvae on standard food and then transferred the larvae to a 1 h conditioning in 20% sucrose solution containing Coomassie blue (stains hind-gut) and fluorescent Eosin (stains fore-gut). After the hour, we scored the larvae for food consumption (Fig. 3a), and we quantitated carbohydrate levels. In this assay, the hyperglycemic, hypertrehalosemic P-element mutant for Mio, fly homolog of mammalian ChREBP, larvae demonstrated a strong feeding aversion and an NN (N for Coomassie, N for Eosin) phenotype, as illustrated by the absence of both dyes in their intestine (Fig. 3b, c). Mutants for insulin pathway-related (Chico, Glut1), MODY (maturity onset diabetes of the young) homologs (HNF4, Hex), and glycolysis genes (Hex, Pgi, Pfk, Ald, Pglym78, Eno, and Pyk) showed increased glucose levels relative to controls, ranging from 1.5-fold to 4.5-fold, but variable trehalose levels from low to high (0.7-fold to 1.6-fold) (Fig. 3d, e). In these studies, food consumption inversely correlated with glucose levels, but did not track with trehalose levels (Fig. 3d, e). These results, using a visual food aversion assay, were similar to our findings with the quantitative assay.
Metabolic mutants display reduced sucrose feeding. a Schematic of the qualitative dual-dye liquid feeding assay. Larvae were scored for dye ingestion, relative to controls, after 1 h on 20% sucrose supplemented with Coomassie and Eosin dyes. Eosin dye was added at 50 min. b Hyperglycemia and hypertrehalosemia detected in Mio mutant larvae. c Microscopic image of Coomassie blue and Eosin dyes in w1118 control and Mio mutant guts after 1 h culture in 20% sucrose. d, e Glucose (d), trehalose (e), and two-dye sucrose feeding (d, e) were scored for key proof-of-principle mutants (insulin pathway- Glut1, Chico; MODY- Hex-C, HNF4; Glycolysis- Pyk, Eno, Pgi, Pfk, Ald, Pglym78). n = 4 each (≥10 larvae per glucose/trehalose replicate). ≥30 larvae per feeding sample. Error bars indicate s.d. Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001
In our next approach, we evaluated feeding in collections of RNAi knockdown flies that we had previously shown to have altered levels of glucose and trehalose24. As a step, we selected three strong “flyabetes” hits Mio, Ck1alpha, and Uba1 that we had identified in our previously reported “Glucome” screens24. Fat body loss-of-function of all three candidates produced extreme sucrose aversion (NN) and hyperglycemia: a 4-fold, 11-fold, and 14-fold glucose increase in MioRNAi, Ck1alphaRNAi, Uba1RNAi larvae, respectively (Fig. 4a). Yet, only MioRNAi larvae had high trehalose (1.8 fold), while, Ck1alphaRNAi and Uba1RNAi had reduced trehalose levels to 0.22-fold and 0.5-fold, respectively. Un-induced RNAi larvae for Mio, Ck1alpha, and Uba1 had control levels of carbohydrates, and fed normally (Supplementary Fig. 2). These data mirror the observations with the various diets and P-element mutants.
Hemolymph glucose elevations induce satiety behaviors. a Glucose (left) and trehalose (right) levels, and two-dye sucrose feeding was measured in w1118; Dcg-Gal4 control and “flyabetic” larvae; dotted line indicates control concentrations. b, c Glucose (b), trehalose (c)24, and two-dye sucrose feeding (b, c) were concurrently assayed in 14 other hyperglycemia candidates (fat body knockdowns), and plotted relative to controls levels. n = 4 each (≥10 larvae per glucose/trehalose replicate). ≥30 larvae per feeding sample Error bars indicate s.d. Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001
We next simultaneously analyzed glucose, trehalose, and feeding using the dual dye assay with additional 14 flyabetes candidates, from the aforementioned RNAi screens24. Larvae with the highest glucose levels did not eat (N); those with intermediate glucose elevations often displayed moderate aversion (P), while candidates that were not significantly hyperglycemic in this instance fed normally (Y) (Fig. 4b, c). Remarkably, out of a total of 11 hyperglycemic food-averse candidates, 5 had normal trehalose, and 5 had lower trehalose. Although this is only an association, together these data suggest that circulating glucose, but likely not trehalose, may function as a signaling metabolite and may have important roles in appetite control.
Hyperglycemic larvae show lower motivation to work for food
Foraging (active food seeking behaviors) is another established and independent indicator of appetite16,17,18. To further evaluate the notion that glucose might control appetite, we analyzed the foraging behavior of flyabetes hits Ck1alphaRNAi, MioRNAi, Uba1RNAi (Fig. 5a, b, Supplementary Fig. 3a). We placed RNAi, and control (w1118; Dcg-Gal4) larvae on Coomassie-supplemented 0.5% (low) agar food plates (Fig. 5c). While most w1118 larvae burrowed deep inside the food and resumed feeding within the first hour, all hyperglycemic larvae lingered on the food surface and many wandered away from the food to the plate lid (Fig. 5d, e). After 3 h, larvae were analyzed for food ingestion; Ck1alphaRNAi, MioRNAi, and Uba1RNAi larvae contained ~35%, 50%, and 85% less food, respectively, relative to those of controls (Supplementary Fig. 3c).
Hyperglycemic larvae have abnormal foraging behaviors. a w1118; Dcg-Gal4 control and flyabetic larvae reared on standard food were provided Coomassie dye-supplemented 20% sucrose and after 1 h feeding was assayed. b Relative dye ingested in 1 h of 20% sucrose feeding was visually detected in control and flyabetic larvae. c w1118; Dcg-Gal4 control and flyabetic larvae (n = 100 each) were transferred to Coomassie-dyed food plates, and initiation of foraging was monitored. d, e Burrowing (d) and foraging (e) activity of control and flyabetic larvae on 0.5% agar media was observed 2 h after transfer. 100 larvae transferred to each agar plate. Number of larvae not yet inside the food is indicated on the bottom left corner of each panel (e). Error bars indicate SEM. Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001
Solid food is generally less attractive to larvae16 and difficulties getting to the food source may be a barrier to feeding. Therefore, we also tested foraging activity on minimally resistant 0.2% agar media and agar-free yeast-sucrose paste; however, lowering resistance did not persuade hyperglycemic larvae into the food (Supplementary Fig. 3d-e). Collectively, these data indicate that hyperglycemic larvae have reduced drive to obtain food.
Hyperglycemic larvae display a general aversion to feeding
The studies described above indicate that hyperglycemic larvae have a reduced interest in eating calorific high sugar. To assess if diminished appetite was more general, we transferred hyperglycemic larvae 20% sucrose-reared w1118 and MioRNAi, Ck1alphaRNAi, Uba1RNAi, and controls, to water, 2.5% Splenda, 5% sucrose, 20% sucrose, and soluble 1% yeast (high protein) solutions containing Coomassie and Eosin dyes, for 1 h (Fig. 6a). Controls reared on 5% sugars ate all food media, but the amount ingested was much greater in the presence of yeast (Y + Y + ) (Fig. 6b, c). w1118 HS and RNAi larvae consumed little to none of the water, Splenda, and sucrose (Fig. 6b). Of note, we sometimes observed a partial rescue of the feeding aversion in all hyperglycemic larvae, except Uba1RNAi, when media contained proteinaceous yeast (Fig. 6a, b), indicating that protein sensing may induce a higher drive to eat. However, RNAi larvae transferred to a solution of Coomassie-protein aggregates for 2 h ate almost none of the protein (Fig. 6d), in contrast to euglycemic controls that left behind a clear solution. These data imply that increases in circulating glucose induce a general rather than a carbohydrate-specific feeding suppression.
Food aversion by hyperglycemic larvae is not limited to sucrose. a w1118 control, w1118 high sugar-reared larvae, and flyabetic larvae (reared on standard food) were provided water, 2.5% Splenda, 5% sucrose, 20% sucrose, and 1% yeast solutions containing Coomassie and Eosin dyes for 1 h. b Larval guts were scored for the presence Coomassie and Eosin dyes after 1 h on various media. ≥30 larvae per treatment. c Relative amounts of 5% sucrose and 1% yeast ingested in 1 h by w1118 control larvae reared on standard food. n = 4 each (20 larvae per replicate). d w1118 control and flyabetic larvae were transferred to a solution containing insoluble Coomassie blue-protein aggregates (CM) and allowed to feed for 2 h. (i) Coomassie-protein aggregate solution in well, before addition of larvae (ii). Larvae just added to solution (t = 0). e Solutions were pictured 2 h later, following removal of control and flyabetic larvae. L = Larvae left behind. Un-ingested Coomassie-protein speckles are visible in solutions, post-feeding by RNAi larvae. f Larvae were separately photographed at 2 h. Error bars indicate s.d. Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001
Lowering glucose in flyabetic larvae triggers re-feeding
Most flyabetic flies are slightly developmentally delayed but survive to adulthood. Unsurprisingly, Ck1alphaRNAi, MioRNAi, and Uba1RNAi larvae reared on Coomassie-supplemented standard food all contain gut dye but in highly variable amounts, (Supplementary Fig. 4a, b) which may be explained by intermittent rather than continuous food consumption. To explore if RNAi larvae resume feeding in the acute setting, and if this is accompanied by changes in circulating carbohydrates, we transferred Ck1alphaRNAi and MioRNAi larvae to 5% sucrose containing Coomassie for 3 h, and then separated feeding (dye in gut) from as yet non-feeding larvae (no dye in gut) (Fig. 7a, b). Remarkably, we found that flyabetic larvae that ate had lowered their glucose levels to that of controls, but those that remained food averse, still had 5-fold- (Ck1alphaRNAi) and 9-fold-increased (MioRNAi) glucose levels (Fig. 7c). In contrast, the trehalose status of all groups was unchanged (Fig. 7c). In a subsequent experiment, we transferred Ck1alphaRNAi, MioRNAi, and control larvae to Coomassie-dyed 5% sucrose solution and monitored changes in glucose and percent larvae feeding between 2 h to 4 h post-transfer. In a subsequent experiment, average glucose levels of flyabetic larvae sampled between 2–4 h post-transfer to sucrose decreased with time, while the proportion of actively feeding larvae increased (Supplementary Fig. 4c). These data suggest that flyabetic larvae are a mix of glucose and feeding states.
Larvae resume feeding on alleviation of hyperglycemia. a w1118; Dcg-Gal4 control and flyabetic larvae (≥75 larvae each) were cultured in 5% sucrose solution for 3 h, after which glucose and trehalose were measured in feeding and non-feeding flyabetic larvae, and compared to controls. b Feeding and non-feeding flyabetic larvae imaged at 3 h. c Relative glucose (left) and trehalose (right) levels, compared to feeding controls, were measured in feeding and non-feeding larvae. d Relative glucose (left) and trehalose (right) levels, and two-dye sucrose feeding were compared in control and Ck1alphaRNAi larvae chronically cultured in the presence or absence of trehalase inhibitor drug. e Glucose levels were measured and two-dye sucrose feeding observed in controls and Ck1alphaRNAi larvae cultured in the presence or absence of Phlorizin. n ≥ 3 each (≥10 larvae per glucose/trehalose replicate). ≥30 larvae per feeding sample. Error bars indicate s.d. Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001
To test if pharmacologically reducing glucose levels could alleviate feeding aversion, we reared control and CK1alphaRNAi larvae in standard food containing vehicle or 25 µM of drug Deoxynojirimycin hydrochloride (Cayman Chemicals, # 10011718), which inhibits trehalase, an enzyme required for catalysis of trehalose to glucose26,27,28. We found that in CK1alphaRNAi drug-treated larvae, glucose levels were reduced (7 to 3-fold) and trehalose levels increased (0.2 to 0.5-fold), and they had improved feeding relative to untreated CK1alphaRNAi larvae (Fig. 7d). We also evaluated glucose and feeding changes in Ck1alphaRNAi and control larvae cultured on food containing 10 mM Phlorizin, a drug known to interfere with mammalian glucose transport from the gut into the circulation, and demonstrated to be also effective in flies28. We observed that Phlorizin alleviated hyperglycemia, and partly rescued the feeding defect in treated Ck1alphaRNAi larvae; glucose and feeding (qualitative and quantitative) was unaffected in Phlorizin-receiving controls (Fig. 7e, Supplementary Fig. 4d, e). These findings support the notion that hemolymph glucose functions as a signaling molecule and a cue in Drosophila larvae, and thereby controls feeding.
Insulin signaling and SLC5A11 may regulate larval feeding
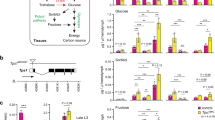
Several studies have demonstrated that fly insulins are secreted in response to elevated hemolymph sugars19,29, and can inhibit motivated feeding in fasted larvae16. To test if elevations in insulin-like peptides might be part of the mechanism for blunted feeding, we measured and observed DILP2 and DILP3 mRNAs were increased in MioRNAi,Ck1alphaRNAi, and Uba1RNAi larvae (Fig. 8a); interestingly, in the latter two, total circulating sugars (glucose plus trehalose) are lower than controls, indicating that glucose elevations alone are sufficient to promote insulin expression. Further, the expression of DILP6, a fat-body derived peptide known to negatively modulate IPC insulin expression30,31,32 was lower in all three flyabetic larvae (Fig. 8a). To evaluate if and how systemic insulin signaling may be affected in flyabetic larvae, we detected transcripts of tobi (target of brain insulin) and Thor (4EBP), which are repressed and induced, respectively, by active peripheral insulin signaling30,33. We found that flyabetic larvae had increased tobi but decreased Thor mRNA levels, relative to controls, suggesting that insulin signaling was upregulated (Fig. 8b). Next we tested loss- and gain-of-function lines for many glucose sensor candidates such as fructose sensor Gr43a34, sugar transporter-like sut3, anorexia gene Gr28b35, fat body GPCR BOSS36, receptors for NPF, sNPF, and dopamine etc. for larval feeding defects. Only larvae lacking expression of SLC5A11, a sodium/solute co-transporter–like protein, showed consistently decreased sucrose feeding. Further, SLC5A11 transcript levels were significantly reduced in all three Ck1alphaRNAi, MioRNAi, and Uba1RNAi flyabetic larvae (Fig. 8c, d). A recent study in adult flies, found that SLC5A11 is used to detect the nutrient value of food, and is expressed in the ellipsoid body of the brain; silencing of SLC5A11-expressing neurons effectively decreases feeding28,37.
Insulin signaling and SLC5A11 (a sodium/solute co-transporter-like protein in flyabetic larvae) may be involved in glucose-responsive feeding behavior. a Relative levels of insulins DILP2, DILP3, and DILP6 expressed in Ck1alphaRNAi, MioRNAi, and Uba1RNAi flyabetic larvae. n ≥ 3 each (20 larvae per replicate). b mRNA levels of insulin targets tobi and Thor in flyabetic larvae, relative to controls. c SCL5A11 fold change in flyabetic larvae, relative to controls. n ≥ 3 each (20 larvae per replicate). d Relative glucose and Coomassie-dye sucrose feeding is indicated in larvae with altered insulins/ insulin signaling, and SLC5A11, relative to controls. n ≥ 3 each (≥10 larvae per glucose replicate). ≥30 larvae per feeding sample. e, f Glucose levels (e) and feeding (e, f) were assayed in Ck1alphaRNAi larvae cultured in the presence or absence of PI3K inhibitor LY294002. n = 4 each (≥10 larvae per glucose replicate). ≥30 larvae per feeding sample. g, h Glucose (g) and feeding (g, h) were compared in Mio mutants with enhanced excitation of neuronal SLC5A11, relative to Mio flyabetic larvae. n = 4 each (≥10 larvae per glucose replicate). ≥30 larvae per feeding sample. (i) Relative expression of tobi and Thor in SLC5A11 loss-of-function larvae, and SLC5A11 mRNA levels in larvae with reduced DILPS or activated insulin signaling. n = 3 each (20 larvae per replicate). Error bars indicate s.d. Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001
Next we probed if insulin signaling and SLC5A11 may be involved in feeding responses of flyabetic larvae. We found that larvae with reduced expression of key DILPs fed normally on Coomassie-dyed 20% sucrose despite hyperglycemia, while larvae with activated insulin signaling were euglycemic but did not eat (Fig. 8d, Supplementary Fig. 5a). The latter observation was also true for SLC5A11 mutants that had no detectable dye in their gut despite having normal glucose levels (Fig. 8d). We hypothesized that alleviation of insulin signaling in flyabetic larvae may improve feeding; hence, we cultured Ck1alphaRNAi and MioRNAi, and controls, in food containing 100 µM of LY294002, a PI3K inhibitor drug (Cayman Chemicals, #70920), or DMSO. We found that while glucose levels remained high in both treated and untreated flyabetic larvae, they consumed notably more sucrose in the 2nd hour of acute sucrose feeding (Fig. 8e, f, Supplementary Fig. 5b, c). The drug did not alter glucose or feeding in controls. Next, to test if ectopic SLC5A11 expression in flyabetic larvae can at least partially reverse their feeding defect, we caused hyper-excitation of neuronal SLC5A11 in a Mio mutant by expressing a voltage gated bacterial Na + channel, and alternately inhibited K + channel KCNQ expression in Mio mutants to mimic SLC5A11 overexpression38, using central nervous system (CNS) driver Elav-Gal4, respectively. SLC5A11 expression in Mio mutants did not significantly affect glucose levels but improved feeding, detectable after 1 h on dyed 20% sucrose (Fig. 8g, h). Additionally, we attempted and succeeded in a partial rescue of feeding in MioRNAi larvae (Supplementary Fig. 5e, f), however the presence of dual drivers (Dcg-Gal4 and Elav-Gal4) in the ‘rescued’ larvae likely contributes to the effects on glucose and feeding.
Finally to begin understanding the interaction of insulin signaling with SLC5A11 in glucose-mediated feeding, we measured tobi and Thor mRNA in SLC5A11 loss-of-function flies, and SLC5A11 mRNA in flies lacking DILPs. While tobi and Thor levels remain unchanged in (Df) SLC5A11 larvae, SLC5A11 expression was upregulated 6-fold in larvae with reduced DILPs expression but lower by 35% in flies with neuronal activation of insulin signaling, suggesting that SLC5A11 may lie downstream of insulin signaling (Fig. 8i). Therefore, it appears that SLC5A11 levels in flies may be regulated by insulin signaling, and SLC5A11 may play a central role in modulating feeding behaviors in response to glucose-sensitive insulin signaling.
Microarray highlights other feeding behavioral candidates
To identify additional factors that may contribute to glucose sensing, we conducted global microarray screening on Ck1alphaRNAi and control lines (Supplementary Fig. 6a). These new data underscored the role of SLC5A11 and insulin signaling in diabetic, non-feeding larvae (Table 1). SLC5A11 was down-regulated by >70% in diabetic, non-feeding Ck1alphaRNAi larvae once again suggesting its involvement in glucose sensing. Loss of Ck1alpha also affected insulin signaling: dilp6, antagonist of brain insulins30,31,32, and ImpL2, a negative regulator of insulin signaling39, were reduced by >80% and >50%, respectively. Tobi, induced by insulin30, was up-regulated almost 5-fold, supporting our earlier QPCR data indicating elevated insulin signaling in flyabetic larvae.
Additionally, the microarray revealed downregulation of AkhR (0.13 fold), the receptor of fly glucagon Akh. Expressed in the fat body and gustatory neurons, loss of AkhR reduces food intake in flies29,33,40. Interestingly, the CCHamide-1 receptor (CCHa1-R), required for starvation-induced olfactory responses in adult41, also showed decreased expression (0.5 fold). The function of CCHa1-R and its peptide ligand CCHamide-1 is yet unknown, but its relative CCHamide-2 promotes feeding and release of IPC insulins in larvae42. We confirmed lower transcript levels of AkhR and CCHa1-R in all three flyabetic Ck1alphaRNAi, MioRNAi, Uba1RNAi larvae by QPCR (Supplementary Fig. 6b). In addition to the above factors, the microarray revealed moderate changes in a number of genes associated with (mostly adult) fly feeding behaviors, glucose/sugar homeostasis and transport, and taste perception (Table 1). However, feeding was not altered in loss- and gain-of-function lines for several of these genes (e.g. fructose sensor Gr43a) individually tested during the screening process. Collectively, our data suggests that insulin signaling and the SLC5A11 sodium/solute co-transporter like protein are key in regulating feeding in flyabetic larvae.
Feeding screen identifies glucose homeostatic regulators
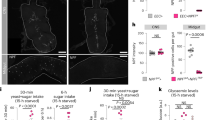
To explore the potential of the dual dye qualitative feeding assay to identify satiety and hyperglycemia hits in a high throughput manner, we interrogated 2274 randomly selected P-element mutants (Fig. 9a). We screened for NN larvae (<50% feeding of each dye), and identified 237 (10.4%) hits using these criteria. Among these, we selected 63 lines that fed most poorly (<10% feeding) and quantified their hemolymph glucose levels (Fig. 9a). We found that 30 of the 63 food-averse larvae (~50%) were hyperglycemic (Fig. 9b, Supplementary Table 1); none were significantly hypoglycemic. This is a much higher percentage than we previously observed in our fly glucome screening of randomly selected genes (~9%)24, supporting the notion that food averse larvae are enriched in hyperglycemic flyabetes larvae. 22 of the 30 hyperglycemia genes had mammalian homologs, and among these 13 had no known link to glucose regulation in database screening done as previously described24 (Fig. 9c, Supplementary Table 1). Together, these data indicate that the fly is a rich source to identify genes that control feeding behavior and those with “satiety” are biased to glucose elevations.
Food aversive mutants identified in sucrose satiety screen are often hyperglycemic. a Larvae from >2000 P-element mutant lines were screened for feeding abnormalities using the qualitative dual-dye liquid feeding assay, and those with severe feeding aversion were assayed for glucose. ≥30 larvae per feeding sample. ≥10 larvae per glucose replicate, n ≥ 3 each. b 30 of 63 P-element “satiety” mutants assayed for glucose levels were found to be hyperglycemic. c Of the 30 candidates, the number with mammalian homologs, and glycemia-linked roles and novel genes are indicated. Error bars indicate s.d. Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
The ability to maintain a relatively constant metabolic milieu is a conserved feature of single and multicellular organisms10,13,43. To ensure energy homeostasis, a host of mechanisms, many of which are conserved, have been deployed38,44,45,46,47,48. Carbohydrates, such as glucose, are a key source of energy for cells and organisms, and also have important signaling roles7,38,43,46,47. Perturbations in glucose availability, consumption, and energy balance due to genetic defects or calorie overload can trigger the onset of metabolic diseases such as type 2 diabetes2,3,4,5,49. The delineation of roles that carbohydrates and glucose play in metabolic homeostasis, therefore, may have broad implications.
In Drosophila, the disaccharide trehalose is present at much higher levels than glucose; many fly interrogations have therefore centered on its metabolic functions45,50,51. Recent studies have highlighted glucose as another important regulatory carbohydrate24,25,52,53,54,55; environmental challenges, excess nutrients, or key genetic mutations can produce significant increases in hemolymph glucose, without concurrent changes in trehalose levels24,25. Chronic hyperglycemia can lead to abnormalities in Drosophila kidney and cardiac function that resemble aspects of the organ failures in mammalian diabetes56,57. Drosophila larvae maintain consistent carbohydrate levels despite their relatively continuous feeding activity24,58, thus, they represent a distinctive model to uncover key homeostatic mechanisms and to interrogate potential behavioral changes in response to glycemic disturbances.
Feeding behavior is a critical component of energy balance10,13. We found that “wild-type” w1118 larvae reared on high sugar (20%) had elevated hemolymph glucose and trehalose, and consumed less food in acute settings compared to counterparts reared on standard media (5% sugars); however, acute exposure to high sucrose was sufficient to increase hemolymph glucose and to decrease feeding rates, though trehalose remained unaffected. These studies indicated that the reduced feeding appeared linked to hemolymph glucose, rather than trehalose, levels. Basal glucose levels in Drosophila larvae are relatively low, making detection of hypoglycemia and its effects on baseline feeding rates difficult. Yet, we observed slightly lower glucose levels, unchanged trehalose, but mild upregulation of feeding rates in w1118 larvae chronically cultured in lower (1%) sugar. Next, we examined the feeding behavior of a variety of fly metabolic mutants and flyabetes candidates24. Larvae that were averse to consuming sucrose had high glucose levels but variable trehalose levels. Interestingly, in all our screening, we did not isolate larvae with consistently elevated trehalose levels, but normal glucose levels; hypertrehalosemic larvae are almost always hyperglycemic as well. On the other hand, larvae with hyperglycemia can be hypotrehalosemic, eutrehalosemic, or hypertrehalosemic. Thus, glucose elevations, apparently independently of circulating trehalose levels, seem to mediate suppression of sucrose feeding in mid-third instar larvae.
Drosophila larvae exert different levels of effort to obtain food that correlate with their level of hunger16,18. Larvae transferred to fresh semi-solid media must make a renewed effort to penetrate the food surface and commence eating16,17,18. We noted that markedly hyperglycemic larvae appeared to have diminished motivation to burrow inside low resistance media, taking at least twice as long as controls to initiate foraging, which may indicate abnormal satiety. Such behaviors are quite unusual; typically mid-third instar larvae forage and feed continuously52,59. These data are consistent with the possible presence of a sensitive internal cue that detects increases in hemolymph glucose and mediates suppression of foraging and feeding. In support of this notion, we observed that these larvae eventually resume feeding upon restoration of euglycemia. Pharmacological lowering of glucose levels similarly ameliorated feeding behavior. These decisions appear linked to glucose, as we did not observe a correlation between trehalose changes and re-initiation of feeding.
Feeding decisions in flies are guided by palatability and nutritional value of the food, though taste sensing and calorie sensing function independently48,60,61. Yet, the presence or absence of carbohydrates or the level of gustatory stimulation of the diet did not appear to influence the feeding behavior of hyperglycemic larvae, supporting the possibility that hyperglycemia may induce a general suppression of feeding. Though, some non-feeding hyperglycemic larvae responded to the presence of yeast, a source of a variety of nutrients and high protein62,63. These results indicate the possible presence of systems for the recognition and ingestion of protein, consistent with previous suggestions that protein sensing occurs independently from carbohydrate sensing and that hemolymph amino acid levels may regulate protein feeding through peripheral and internal receptors63,64,65. We also observed increased (wild type) larval feeding on non-nutritive Splenda (sucralose) than sucrose. It has been reported that adult flies display a greater ingestion response (preference index, PI) to agar containing sucralose (PI = 90%) than sucrose (PI = 52%)66, suggesting that flies may find sucralose innately more appetitive. Moreover, hungry animals may simply eat more of the super-sweet calorie-free diet to induce satiation. A recent study showed that sucralose increases food intake in flies via a neuronal fasting response comprising the NPF system67.
One potential mechanism of carbohydrate sensing may be through induction of expression of insulin-like peptides, DILP2 and DILP3 associated with hemolymph sugar homeostasis52,68. Numerous investigations in insects and mammals have indicated a central role of insulin in feeding regulation, possibly through complex interactions with neuromodulators such as NPF/NPY, dopamine, serotonin etc2,16,18,44,62,65. In support of these findings, we observed that flyabetic larvae had increased DILP2 and DILP3 expression. Thus glucose-mediated suppression of feeding in hyperglycemic larvae may occur, at least in part, via increased insulin activity; altered expression of insulin targets, tobi and Thor indicated increased peripheral insulin signaling. Further, hyperglycemic larvae deficient in key insulins (DILPs2, 3, 5, and 6) fed normally, while euglycemic larvae with activated insulin pathway ate less. Pharmacological inhibition of insulin signaling by a PI3K inhibitor partially rescued feeding in flyabetic larvae. Our data also support the notion of an internal cue/sensor capable of detecting increases in hemolymph glucose or neuropeptides, and mediating feeding suppression. One such candidate SLC5A11, a member of the sodium/solute co-transporter family, showed lower expression in flyabetic larvae. In flies, SLC5A11 is upregulated during starvation, and promotes hunger-driven food intake37. Neuronal activation of SLC5A11 partially reversed the feeding defect in flyabetic larvae, similar to the effect from suppressing insulin signaling, suggesting its possible involvement and interaction with the insulin pathway in mediating glucose-regulated feeding. QPCR and feeding data suggests that SLC5A11 may function downstream of insulin signaling, and may serve on the pathway of glucose sensing. Sustained hyperglycemia and hyperinsulinemia resulting from loss of key glucose regulatory genes or chronic high sugar diet may induce transcriptional and other irreversible changes. Indeed decrease in SLC5A11 expression was more pronounced in larvae cultured in 20% sugars relative to those acutely fed a high sucrose diet (Supplementary Fig. 7); this may explain the enhanced severity of the feeding defect in the former versus the latter, despite similar glucose levels (Figs. 1b, c, 2b, c).
Finally, the qualitative dual-dye feeding assay may be useful as an additional high throughput screening tool for satiety and Glucome candidates since feeding defective hits appear to be enriched in glucose regulators. Larvae may alter feeding independently of hemolymph glucose concentrations for other reasons, for example, mutations producing anatomical defects69,70, or affecting genes in non-glucose regulated satiety pathways22,23, or downstream of glucose sensing. Of note, it is easier and more time- and resource-efficient to first screen for feeding using the dual dye assay followed by glucose quantitation compared to glucose quantitation alone. Thus, the coupled screening approaches are appropriate to find genes important in appetite and glucose homeostatic control, two potential keys to the global epidemics of obesity and diabetes.
In summary, we investigated a potential relationship between circulating carbohydrate levels and feeding behavior in Drosophila mid-third instar larvae. We found that high hemolymph glucose levels cause a general, not just sugar-specific, suppression of feeding. Further, hyperglycemic larvae display reduced motivation to work for food, hinting at unusual satiation. Despite its considerable dominance in hemolymph, trehalose levels did not appear to influence larval feeding or foraging. While insulins and SLC5A11 appear to be key players in glucose-responsive feeding, it is quite likely that other cumulative changes, triggered by the hyperglycemia, contribute to the observed feeding defect; in the fly there is substantial and complex crosstalk occurring between the brain and non-IPC insulins, Akh, NPF, sNPF, hugin, drosulfakinin, dopamine etc33,45,48. Our microarray data suggests that Akh signaling may be down-regulated in flyabetic larvae. Akh has orexigenic properties, and controls expression of NPF and CCHamide-2, as well as DILP2, 3, and 6. This system is likely just one of many as energy balance is critical to organismal survival, and feeding decisions involve additional inputs and complexities10,12,13. Fruit flies and hyperglycemic non-feeding larval may offer opportunities to dissect some aspects of feeding behavioral mechanisms, potentially ones which are evolutionarily conserved, that regulate hunger, feeding, and satiety. Appetite control is an important contributor to obesity, and diabetes progression1,3,6. Further dissection of the glucose sensing machinery and the role of glucose in appetite signaling cascades may illuminate mechanisms that govern feeding decisions in fly larvae that may also function in mammals.
Methods
Fly strains and culture conditions
Flies were reared on standard cornmeal-molasses-yeast-agar food. All UAS-dsRNA (RNAi), P-element, deficiency, and other transgenic strains were obtained from the Bloomington Stock Center. Dcg-Gal4 flies were described24. High sugar media was prepared by adding sucrose (15 g/100 ml) to standard food. This conditioning media also contains a host of other ingredients and caloric sources, for example, yeast and cornmeal. All flies including RNAi loss-of-function crosses were maintained at 25 °C. The common laboratory strain w1118 was used as the control line. In experiments involving RNAi, progeny from the crosses between paternal w1118 and maternal Gal4 driver served as controls. To account for developmental delays, culturing of w1118 larvae on 20% sugars was started 1-2 days before those on 1% and 5% sugars. Ck1alphaRNAi and Uba1RNAi cultures were started 1day before MioRNAi and controls (Dcg-Gal4 > w1118).
Larval collection
After egg laying, larvae were cultured in medium to high-density conditions (75-150 larvae/vial) for 6–7 days before harvesting feeding mid-third instars. Vials were rid of older wandering larvae on the sides and food surface. Larvae were collected in 630 µm mesh-fitted baskets (Genesee) and washed to get rid of adherent food particles. Baskets were lowered into a tray of water to allow dead flies to float out, and then into a shallow tray of 2 M NaCl so that larvae float to the top. Larvae were transferred with a paintbrush to Pyrex 9 depression glass spot plates (Corning), and used for different assays.
Glucose and trehalose assays
Larvae were divided into 4 piles (10–12 larvae each) on a strip of parafilm. Larvae were bled by tearing the cuticle with Dumont 5 forceps (Electron Microscopy Sciences). Two microlitres of colorless hemolymph was aspirated from each pile and separately transferred to 96-well plates (Thermo-Scientific) containing 0.1% N-Phenylthiourea (Sigma-Aldrich) in 50 µl PBS. 150 µl of Autokit Glucose reagent (Wako) was added to each well, and incubated at room temperature for 20 min before measuring absorbance at 505 nm. Glucose concentration was calculated from a standard curve generated with manufacturer’s glucose standards. For trehalose assays, 8 µl of dilute hemolymph was treated with 5 µl of (diluted 8X) porcine kidney trehalase (Sigma) overnight at 37 °C. Ten microlitres of treated sample was assayed for trehalose as described for glucose.
Liquid feeding assay
Duplicate samples of 30–50 larvae were transferred to 24-well plates containing 250 µl of 20% sucrose + Coomassie blue dye (200 µg/ml). After 50 min, 10 µl of 0.5% Eosin Y (Sigma) fluorescent dye was added to each well. Ten minutes later (total 1 h) larvae were removed and rinsed in water. Through a dissecting scope, larval guts were scored for the presence of Coomassie blue and Eosin dyes using white light and TRITC filter, respectively. The dual dyes allow us to visualize and to quantitate if food consumption might be altered. Larval food consumption was scored as follows: “Yes” (Y) if > 90% of larvae contained dye at levels approximating w1118 controls; “Partial” (P) if between 50–90% of larval guts had any dye, and/or > 90% larvae had dye but at very low levels; “No” (N) if < 50% of larvae had eaten.
Soft agar response assay
Soft agar food was prepared with 5% sucrose, 10% yeast, and 0.5% or 0.2% agar, otherwise nutritionally similar to standard fly media Agar was melted in the microwave and Coomassie dye (200 µg/ml) was added to allow easy visualization of the whitish-yellow-hued larvae, and quantification of food ingestion. Agar was poured into 5 cm Petri plates to form a ~5 mm thick layer. Once dry, 100 larvae were introduced to the center of the agar plate and monitored for foraging activity.
Insoluble protein feeding assay
150 larvae were transferred to 24-well plates containing 400 µl of 5% yeast extract solution. Addition of Coomassie dye, prepared in water instead of ethanol, to 5% yeast extract produces insoluble blue dye-protein complexes. After 2 h, larvae were removed, rinsed in water and then methanol. Both wells and larvae were photographed to record dye-protein ingestion.
Food ingestion quantification
Larvae fed Coomassie dye-supplemented media were harvested, washed in water and methanol, blotted dry, and transferred to 1.5 ml Eppendorf tubes on ice (30 larvae/sample, in triplicate). Larvae were homogenized in 200 µl of 100% methanol. Samples were centrifuged at 14,000 rpm for 10 min; 175 µl of the supernatant was transferred to fresh tubes containing 175 µl of dH2O, and centrifuged again. Absorbance of this supernatant was measured at 595 nm, and dye amount (food ingested) was calculated by comparing with a standard curve generated from a range of Coomassie dye concentrations.
Quantitative RT-PCR and microarray
RNA for Quantitative RT-PCR was extracted from mid-third instar feeding larvae using Trizol Reagent (Ambion Life Technologies) as per manufacturer’s instructions. cDNA was synthesized from 1 µg of RNA using ReadyScript™ cDNA Synthesis Mix (Sigma). QPCR was performed on the BioRad CFX96 Real-Time System; data was normalized to endogenous control rp49. RNA for Microarray was extracted from larvae using RNA extraction kit (Qiagen), and sent to the UTSW Genomics and Microarray Core Facility for microarray analyses by Affymetrix GeneChip Array. Primer sequences for all transcripts amplified are listed in Supplementary Table 2.
Statistical analysis
Error bars indicate standard deviation (s.d.). Statistical significance was assessed by two-tailed Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001.
Data Availability
The microarray dataset generated during the current study are available in the GEO repository (GSE116003). All other relevant data is available from the authors on request.
References
Bell, G. I. & Polonsky, K. S. Diabetes mellitus and genetically programmed defects in b-cell function. Nature 414, 788–791 (2001).
Gerozissis, K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur. J. Pharmacol. 585, 38–49 (2008).
Hansen, L. & Pedersen, O. Genetics of type 2 diabetes mellitus: status and perspectives. Diabetes Obes. Metab. 7, 122–135 (2005).
Laakso, M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms. Diabetes Care 33, 442–449 (2010).
LeRoith, D. & Accili, D. Mechanisms of disease: using genetically altered mice to study concepts of type 2 diabetes. Nat. Clin. Pract. Endocrinol. Metab. 4, 164–172 (2008).
Weber, M. B., Twombly, J. G., Venkat Narayan, K. M. & Phillips, L. S. Lifestyle interventions and the prevention and treatment of type 2diabetes. Am. J. Lifestyle Med. 4, 468–480 (2010).
Mayer, J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann. N. Y. Acad. Sci. 63, 15–43 (1955).
Levin, B. E. Metabolic sensors: viewing glucosensing neurons from a broader perspective. Physiol. Behav. 76, 397–401 (2002).
Dunn-Meynell, A. A. et al. Relationship among Brain and Blood Glucose Levels and Spontaneous and Glucoprivic Feeding. J. Neurosci. 29, 7015–7022 (2009).
Woods, S. C., Seeley, R. J., Porte, D. Jr. & Schwartz, M. W. Signals that regulate food intake and energy homeostasis. Science 280, 1378–1383 (1998).
Grozinsky-Glasberg, S., Mazeh, H. & Gross, D. J. Clinical features of pancreatic neuroendocrine tumors. J. Hepatobiliary Pancreat. Sci. 22, 578–585 (2015).
Blundell, J. E. et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control a new formulation. Dis. Model Mech. 5, 608–613 (2012).
Woods, S. C. & Ramsay, D. S. Food intake, metabolism and homeostasis. Physiol. Behav. 104, 4–7 (2011).
Chaput, J.-P. & Tremblay, A. The glucostatic theory of appetite control and the risk of obesity and diabetes. Int. J. Obes. 33, 46–53 (2009).
Woods, S. C. Metabolic signals and food intake. Forty years of progress. Appetite 71, 440–444 (2013).
Wu, Q., Zhang, Y., Xu, J. & Shen, P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc. Natl Acad. Sci. USA 102, 13289–13294 (2005).
Wu, Q. et al. Developmental control of foraging and social behavior by the drosophila neuropeptide Y-like system. Neuron 39, 147–161 (2003).
Wu, Q., Zhao, Z. & Shen, P. Regulation of aversion to noxious food by Drosophila neuropeptide Y– and insulin-like systems. Nat. Neurosci. 8, 1350–1355 (2005).
Xu, J., Li, M. & Shen, P. A G-protein coupled neuropeptide y-like receptor suppresses behavioral and sensory response to multiple stressful stimuli in drosophila. J. Neurosci. 30, 2504–2512 (2010).
Lee, K.-S., You, K.-H., Choo, J.-K., Han, Y.-M. & Yu, K. Drosophila short neuropeptide f regulates food intake and body size. J. Biol. Chem. 279, 50781–50789 (2004).
Ren, G. R. et al. CCHamide-2 Is an orexigenic brain-gut peptide in drosophila. PLoS ONE 10, e0133017 (2015).
Soderberg, J. A. E., Carlsson, M. A. & Nassel, D. R. Insulin-producing cells in the Drosophila brain also express satiety-inducing cholecystokinin-like peptide, drosulfakinin. Front. Endocrinol. 3, 1–13 (2012).
Melcher, C. & Pankratz, M. J. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol 3, e305 (2005).
Ugrankar, R. et al. Drosophila glucome screening identifies Ck1alpha as a regulator of mammalian glucose metabolism. Nat. Commun. 6, 7102 (2015).
Pasco, M. Y. & Leopold, P. High sugar-induced insulin resistance in Drosophila relies on the lipocalin neural lazarillo. PLoS ONE 7, e36583 (2012).
Gruber, F. et al. Suppression of conditioned odor approach by feeding is independent of taste and nutritional value in Drosophila. Curr. Biol. 23, 507–514 (2013).
Thorat, L. J. & Gaikwad, S. M. & Nath, B. B. Trehalose as an indicator of desiccation stress in Drosophila melanogaster larvae: a potential marker of anhydrobiosis. Biochem. Biophys. Res. Commun. 419, 638–642 (2012).
Dus, M., Ai, M. & Suh, G. S. B. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute co-transporter in Drosophila. Nat. Neurosci. 16, 526–529 (2013).
Kim, J. & Neufeld, T. P. Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nat. Commun. 17, 6846, https://doi.org/10.1038/ncomms7846 (2015).
Bai, H., Kang, P. & Tatar, M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 11, 978–985 (2012).
Okamoto, N. et al. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev. Cell. 17, 885–891 (2009).
Zhang, Y. & Xi, Y. Fat Body Development and its Function in Energy Storage and Nutrient Sensing in Drosophila melanogaster. J. Tissue Sci. Eng. 6, 141 (2014).
Galikova, M., Kelpsatel, P., Xu, Y. & Kuhnlein, R. P. The obesity-related Adipokinetic hormone controls feeding and expression of neuropeptide regulators of Drosophila metabolism. Eur. J. Lipid Sci. Technol. 171, 1600138 https://doi.org/10.1002/ejlt.201600138 (2016).
Miyamoto, T., Slone, J., Song, X. & Amrein, H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell 151, 1113–1125 (2012).
Ayres, J. S. & Schneider, D. S. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 7, e1000150 (2009).
Kohyama-Koganeya, A., Kim, Y.-J., Miura, M. & Hirabayashi, Y. A Drosophila orphan G protein-coupled receptor BOSS functions as a glucose-responding receptor: loss of boss causes abnormal energy metabolism. Proc. Natl Acad. Sci. USA 105, 15328–15333 (2008).
Park, Y.-J. et al. Drosophila SLC5A11 mediates hunger by regulating K + channel activity. Curr. Biol. 26, 1–10 (2016).
Seeley, R. J. & Woods, S. C. Monitoring of stored and available fuel by the CNS: Implication for obesity. Nat. Rev. Neurosci. 4, 902–909 (2003).
Honegger, B. et al. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 7, 10 (2008).
Bharucha, K. N., Tarr, P. & Zipursky, S. L. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J. Exp. Biol. 211, 3103–3110 (2008).
Farhan, A. et al. The CCHamide 1 receptor modulates sensory perception and olfactory behavior in starved Drosophila. Sci. Rep. 3, 2765 (2013).
Sano, H. et al. The nutrient-responsive hormone CCHamide-2 controls growth by regulating insulin-like peptides in the brain of Drosophila melanogaster. PLOS Genet 11, e1005209 (2015).
Rolland, F., Winderickx, J. & Thevelein, J. M. Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2, 183–201 (2002).
Vogt, M. C. & Bruning, J. C. CNS insulin signaling in the control of energy homeostasis and glucose metabolism – from embryo to old age. Trends Endocrinol. Metab. 24, 76–84 (2013).
Kim, S. K. & Rulifson, E. J. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431, 316–320 (2004).
Cota, D., Proulx, K. & Seeley, R. J. The role of CNS fuel sensing in energy and glucose regulation. Gastroenterology 132, 2158–2168 (2007).
Guo, X. et al. Glycolysis in the control of blood glucose homeostasis. Acta Pharm. Sin. B 2, 358–367 (2012).
Pool, A.-H. & Scott, K. Feeding regulation in Drosophila. Curr. Opin. Neurobiol. 29, 57–63 (2014).
Deshpande, A. D., Harris-Hayes, M. & Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 88, 1254–1264 (2008).
Isabel, G., Martin, J. R., Chidami, S., Veenstra, J. A. & Rosay, P. AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R531–R538 (2005).
Baker, K. D. & Thummel, C. S. Diabetic larvae and obese flies—emerging studies of metabolism in Drosophila. Cell Metab. 6, 257–266 (2007).
Musselman, L. P. et al. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model Mech. 4, 842–849 (2011).
Park, S. et al. A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genet. 10, e1004555 (2014).
Pendse, J. et al. A Drosophila functional evaluation of candidates from human genome-wide association studies of type 2 diabetes and related metabolic traits identifies tissue-specific roles for dHHEX. BMC Genomics 14, 136 (2013).
Rajan, A. & Perrimon, N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151, 123–137 (2012).
Na, J. et al. A drosophila model of high sugar diet-induced cardiomyopathy. PLOS Genet 9, e1003175 (2013).
Na, J., Sweetwyne, M. T., Park, A. S. D., Susztak, S. & Cagan, R. L. Diet-induced podocyte dysfunction in drosophila and mammals. Cell Rep. 12, 636–647 (2015).
Musselman, L. P. et al. Role of fat body lipogenesis in protection against the effects of caloric overload in Drosophila. J. Biol. Chem. 288, 8028–8042 (2013).
Jiang, C., Baehrecke, E. H. & Thummel, C. S. Steroid regulated programmed cell death during Drosophila metamorphosis. Development 124, 4673–4683 (1997).
Dus, M., Min, S., Keene, A. C., Lee, G. Y. & Suh, G. S. B. Taste-independent detection of the caloric content of sugar in Drosophila. Proc. Natl Acad. Sci. USA 108, 11644–11649 (2011).
Stafford, J. W., Lynd, K. M., Jung, A. Y. & Gordon, M. D. Integration of taste and calorie sensing in Drosophila. J. Neurosci. 32, 14767–14774 (2012).
Lebreton, S., Witzgall, P., Olsson, M. & Becher, P. G. Dietary glucose regulates yeast consumption in adult Drosophila males. Front. Physiol. 5, 504 (2014).
Toshima, N. & Tanimura, T. Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. J. Exp. Biol. 215, 2827–2832 (2012).
Bjordal, M., Arquier, N., Kniazeff, J., Pin, J. P. & Leopold, P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell 156, 510–521 (2014).
Itskov, P. M. & Ribeiro, C. The dilemmas of the gourmet fly: the molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front. Cell Sci. 7, 12 https://doi.org/10.3389/fnins.2013.00012 (2013).
Gordesky-Gold, B., Rivers, N., Ahmed, O. M. & Breslin, P. A. S. Drosophila melanogaster prefers compounds perceived sweet by humans. Chem. Senses 33, 301–309 (2008).
Wang, Q.-P. et al. Sucralose promotes food intake through NPY and a neuronal fasting response. Cell. Metab. 24, 75–90 (2016).
Root, C. M., Ko, K. I., Jafari, A. & Wang, J. W. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145, 133–144 (2011).
Pankratz, M. J. & Hoch, M. Control of epithelial morphogenesis by cell cignaling and integrin molecules in the Drosophila foregut. Development 121, 1885–1898 (1995).
Tetzlaff, M. T., Jackle, H. & Pankratz, M. J. Lack of Drosophila cytoskeletal tropomysin affects head morphogenesis and the accumulation of oskar mRNA required for germ cell formation. EMBO J. 15, 1247–1254 (1996).
Acknowledgements
We are grateful to David Mangelsdorf and Steven Kliewer for use of equipment. We thank the Bloomington Stock Center for fly stocks. We are also grateful to members of the Graff and Henne labs for support, reagents, and insights. This study was supported by the National Institute of Health and the National Institute of Diabetes and Digestive and Kidney Disease grants (R01 DK066556, R01 DK064261, and R01 DK088220) to J.M.G., and by the American Diabetes Association grant (7-12-MN-23) to R.U and J.M.G.
Author information
Authors and Affiliations
Contributions
J.M.G., R.U, P.T., and F.A. conceived, designed, and interpreted the experiments. R.U., P.T., and F.A. performed the experiments. W.M.H assisted with discussions and anlyses. J.M.G. and R.U. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
J.M.G. is a co-founder and shareholder of Reata Pharmaceuticals. The remaining authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ugrankar, R., Theodoropoulos, P., Akdemir, F. et al. Circulating glucose levels inversely correlate with Drosophila larval feeding through insulin signaling and SLC5A11. Commun Biol 1, 110 (2018). https://doi.org/10.1038/s42003-018-0109-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-018-0109-4
This article is cited by
-
Genome-wide associations of human gut microbiome variation and implications for causal inference analyses
Nature Microbiology (2020)
-
Insulin signaling in Drosophila melanogaster mediates Aβ toxicity
Communications Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.