Abstract
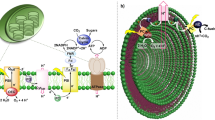
In nature, photosynthetic organelles harness solar radiation to produce energy-rich compounds from water and atmospheric CO2 via exquisite supramolecular assemblies. Although artificial photocatalytic cycles have been shown to occur at higher intrinsic efficiencies, the low selectivity and stability in water for multi-electron CO2 reduction hamper their practical applications. The creation of water-compatible artificial photocatalytic systems mimicking the natural photosynthetic apparatus for selective and efficient solar fuel production represents a major challenge. Here we show a highly stable and efficient artificial spherical chromatophore nanomicelle system self-assembled from Zn porphyrin amphiphiles with a Co catalyst in water for CO2-to-methane conversion with a turnover number >6,600 and 89% selectivity over 30 days. The hierarchical self-assembly induced a spherical antenna effect that could facilitate the photocatalytic process with an initial 15% solar-to-fuel efficiency. Furthermore, it has a capability to efficiently reduce atmospheric CO2 into methane with high selectivity in water.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Barber, J. Photosynthetic energy conversion: natural and artificial. Chem. Soc. Rev. 38, 185–196 (2009).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
van Oijen, A. M., Ketelaars, M., Köhler, J., Aartsma, T. J. & Schmidt, J. Unraveling the electronic structure of individual photosynthetic pigment–protein complexes. Science 285, 400–402 (1999).
Melis, A. Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 177, 272–280 (2009).
Kühlbrandt, W., Wang, D. N. & Fujiyoshi, Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature 367, 614–621 (1994).
McDermott, G. et al. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 374, 517–521 (1995).
Blankenship, R. E. et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 (2011).
Moser, C. C., Keske, J. M., Warncke, K., Farid, R. S. & Dutton, P. L. Nature of biological electron transfer. Nature 355, 796–802 (1992).
Ben-Shem, A., Frolow, F. & Nelson, N. Crystal structure of plant photosystem I. Nature 426, 630–635 (2003).
Bahatyrova, S. et al. The native architecture of a photosynthetic membrane. Nature 430, 1058–1062 (2004).
Şener, M. K., Olsen, J. D., Hunter, C. N. & Schulten, K. Atomic-level structural and functional model of a bacterial photosynthetic membrane vesicle. Proc. Natl Acad. Sci. USA 104, 15723–15728 (2007).
Scholes, G. D., Fleming, G. R., Olaya-Castro, A. & van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 3, 763–774 (2011).
Yang, J., Yoon, M.-C., Yoo, H., Kim, P. & Kim, D. Excitation energy transfer in multiporphyrin arrays with cyclic architectures: towards artificial light-harvesting antenna complexes. Chem. Soc. Rev. 41, 4808–4826 (2012).
Abdi, F. F. et al. Efficient solar water splitting by enhanced charge separation in a bismuth vanadate–silicon tandem photoelectrode. Nat. Commun. 4, 2195 (2013).
Cook, T. R. et al. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110, 6474–6502 (2010).
Luo, J. et al. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 345, 1593–1596 (2014).
Liu, C., Colón, B. C., Ziesack, M., Silver, P. A. & Nocera, D. G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352, 1210–1213 (2016).
Li, X., Yu, J., Jaroniec, M. & Chen, X. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem. Rev. 119, 3962–4179 (2019).
Rao, H., Schmidt, L. C., Bonin, J. & Robert, M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature 548, 74–77 (2017).
Hansen, M., Troppmann, S. & König, B. Artificial photosynthesis at dynamic self-assembled interfaces in water. Chem. Eur. J. 22, 58–72 (2016).
Hansen, M., Li, F., Sun, L. & König, B. Photocatalytic water oxidation at soft interfaces. Chem. Sci. 5, 2683–2687 (2014).
Troppmann, S. & König, B. Functionalized membranes for photocatalytic hydrogen production. Chem. Eur. J. 20, 14570–14574 (2014).
Wang, H.-Y. et al. Photocatalytic hydrogen evolution from rhenium(I) complexes to [FeFe] hydrogenase mimics in aqueous SDS micellar systems: a biomimetic pathway. Langmuir 26, 9766–9771 (2010).
Dumele, O. et al. Photocatalytic aqueous CO2 reduction to CO and CH4 sensitized by ullazine supramolecular polymers. J. Am. Chem. Soc. 144, 3127–3136 (2022).
Tian, J. et al. Tailored self-assembled photocatalytic nanofibres for visible-light-driven hydrogen production. Nat. Chem. 12, 1150–1156 (2020).
Bonchio, M. et al. Hierarchical organization of perylene bisimides and polyoxometalates for photo-assisted water oxidation. Nat. Chem. 11, 146–153 (2019).
Lazarides, T. et al. Photocatalytic hydrogen production from a noble metal-free system based on a water-soluble porphyrin derivative and a cobaloxime catalyst. Chem. Commun. 50, 521–523 (2014).
Behar, D. et al. Cobalt porphyrin catalyzed reduction of CO2. Radiation chemical, photochemical, and electrochemical studies. J. Phys. Chem. A 102, 2870–2877 (1998).
Nganga, J., Chaudhri, N., Brückner, C. & Angeles-Boza, A. M. β-Oxochlorin cobalt(II) complexes catalyze the electrochemical reduction of CO2. Chem. Commun. 57, 4396–4399 (2021).
Boutin, E. et al. Molecular catalysis of CO2 reduction: recent advances and perspectives in electrochemical and light-driven processes with selected Fe, Ni and Co aza-macrocyclic and polypyridine complexes. Chem. Soc. Rev. 49, 5772–5809 (2020).
Boutin, E. et al. Aqueous electrochemical reduction of carbon dioxide and carbon monoxide into methanol with cobalt phthalocyanine. Angew. Chem. Int. Ed. 58, 16172–16176 (2019).
Gonglach, S. et al. Molecular cobalt corrole complex for the heterogeneous electrocatalytic reduction of carbon dioxide. Nat. Commun. 10, 3864 (2019).
Weingarten, A. S. et al. Supramolecular packing controls H2 photocatalysis in chromophore amphiphile hydrogels. J. Am. Chem. Soc. 137, 15241–15246 (2015).
Weingarten, A. S. et al. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat. Chem. 6, 964–970 (2014).
Tian, J. et al. Supramolecular metal–organic frameworks that display high homogeneous and heterogeneous photocatalytic activity for H2 production. Nat. Commun. 7, 11580 (2016).
Faul, C. F. J. & Antonietti, M. Ionic self-assembly: facile synthesis of supramolecular materials. Adv. Mater. 15, 673–683 (2003).
Şener, M. et al. Photosynthetic vesicle architecture and constraints on efficient energy harvesting. Biophys. J. 99, 67–75 (2010).
Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 40, 1305–1323 (2011).
Bonin, J., Chaussemier, M., Robert, M. & Routier, M. Homogeneous photocatalytic reduction of CO2 to CO using iron(0) porphyrin catalysts: mechanism and intrinsic limitations. ChemCatChem 6, 3200–3207 (2014).
Dlugokencky, E. J. & Pieter, T. Trends in Atmospheric Carbon Dioxide (National Oceanic and Atmospheric Administration, Global Monitoring Laboratory, 2019); www.esrl.noaa.gov/gmd/ccgg/trends/
Wu, X. et al. Photocatalytic CO2 conversion of M0.33WO3 directly from the air with high selectivity: insight into full spectrum-induced reaction mechanism. J. Am. Chem. Soc. 141, 5267–5274 (2019).
Sengupta, S. & Würthner, F. Chlorophyll J-aggregates: from bioinspired dye stacks to nanotubes, liquid crystals, and biosupramolecular electronics. Acc. Chem. Res. 46, 2498–2512 (2013).
Rao, H., Bonin, J. & Robert, M. Non-sensitized selective photochemical reduction of CO2 to CO under visible light with an iron molecular catalyst. Chem. Commun. 53, 2830–2833 (2017).
D’Souza, F. et al. Electrochemical and spectroelectrochemical behavior of cobalt(III), cobalt(II), and cobalt(I) complexes of meso-tetraphenylporphyrinate bearing bromides on the .beta.-pyrrole positions. Inorg. Chem. 32, 4042–4048 (1993).
Kornienko, N. et al. Metal–organic frameworks for electrocatalytic reduction of carbon dioxide. J. Am. Chem. Soc. 137, 14129–14135 (2015).
Call, A. et al. Highly efficient and selective photocatalytic CO2 reduction to CO in water by a cobalt porphyrin molecular catalyst. ACS Catal. 9, 4867–4874 (2019).
Sinha, S., Zhang, R. & Warren, J. J. Low overpotential CO2 activation by a graphite-adsorbed cobalt porphyrin. ACS Catal. 10, 12284–12291 (2020).
Hossain, M. N. et al. Temperature-dependent product distribution of electrochemical CO2 reduction on CoTPP/MWCNT composite. Appl. Catal. B 304, 120863 (2022).
Kullmann, M. et al. Ultrafast exciton dynamics after Soret- or Q-band excitation of a directly β,β′-linked bisporphyrin. Phys. Chem. Chem. Phys. 14, 8038–8050 (2012).
Neta, P. & Harriman, A. Zinc porphyrin π-radical cations in aqueous solution. Formation, spectra and decay kinetics. J. Chem. Soc. Faraday Trans. 81, 123–138 (1985).
Brennan, B. J., Durrell, A. C., Koepf, M., Crabtree, R. H. & Brudvig, G. W. Towards multielectron photocatalysis: a porphyrin array for lateral hole transfer and capture on a metal oxide surface. Phys. Chem. Chem. Phys. 17, 12728–12734 (2015).
Shen, J., Kolb, M. J., Göttle, A. J. & Koper, M. T. M. DFT study on the mechanism of the electrochemical reduction of CO2 catalyzed by cobalt porphyrins. J. Phys. Chem. C 120, 15714–15721 (2016).
Zhang, X., Cibian, M., Call, A., Yamauchi, K. & Sakai, K. Photochemical CO2 reduction driven by water-soluble copper(I) photosensitizer with the catalysis accelerated by multi-electron chargeable cobalt porphyrin. ACS Catal. 9, 11263–11273 (2019).
Muresan, N. M., Willkomm, J., Mersch, D., Vaynzof, Y. & Reisner, E. Immobilization of a molecular cobaloxime catalyst for hydrogen evolution on a mesoporous metal oxide electrode. Angew. Chem. Int. Ed. 51, 12749–12753 (2012).
Zhang, X. et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 8, 14675 (2017).
Shen, J. et al. Electrocatalytic reduction of carbon dioxide to carbon monoxide and methane at an immobilized cobalt protoporphyrin. Nat. Commun. 6, 8177 (2015).
Lu, Q. et al. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 5, 3242 (2014).
Yao, C. L., Li, J. C., Gao, W. & Jiang, Q. Cobalt–porphine catalyzed CO2 electro-reduction: a novel protonation mechanism. Phys. Chem. Chem. Phys. 19, 15067–15072 (2017).
Wang, C. et al. An intriguing window opened by a metallic two-dimensional Lindqvist–cobaltporphyrin organic framework as an electrochemical catalyst for the CO2 reduction reaction. J. Mater. Chem. A 8, 14807–14814 (2020).
Guo, L. & Guo, S. Mechanistic understanding of CO2 reduction reaction towards C1 products by molecular transition metal-porphyrin catalysts. Int. J. Hydrog. Energy 46, 10608–10623 (2021).
Pati, P. B. et al. Photocathode functionalized with a molecular cobalt catalyst for selective carbon dioxide reduction in water. Nat. Commun. 11, 3499 (2020).
Arcudi, F., Đorđević, L., Nagasing, B., Stupp, S. I. & Weiss, E. A. Quantum dot-sensitized photoreduction of CO2 in water with turnover number >80,000. J. Am. Chem. Soc. 143, 18131–18138 (2021).
Gong, Y.-N. et al. Regulating photocatalysis by spin-state manipulation of cobalt in covalent organic frameworks. J. Am. Chem. Soc. 142, 16723–16731 (2020).
Zhang, H. et al. Efficient visible-light-driven carbon dioxide reduction by a single-atom implanted metal–organic framework. Angew. Chem. Int. Ed. 55, 14310–14314 (2016).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Warren, S. C. et al. Rapid global fitting of large fluorescence lifetime imaging microscopy datasets. PLoS ONE 8, e70687 (2013).
Manton, J. C., Long, C., Vos, J. G. & Pryce, M. T. Porphyrin–cobaloxime complexes for hydrogen production, a photo- and electrochemical study, coupled with quantum chemical calculations. Dalton Trans. 43, 3576–3583 (2014).
Kosco, J. et al. Enhanced photocatalytic hydrogen evolution from organic semiconductor heterojunction nanoparticles. Nat. Mater. 19, 559–565 (2020).
Mikkelsen, M., Jorgensen, M. & Krebs, F. C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 3, 43–81 (2010).
Acknowledgements
J.T. acknowledges the funding support from National Key Research and Development Program of China (2022YFA1206200); Shanghai Institute of Organic Chemistry and Shanghai Branch, CAS; Shanghai Rising-Star Program (22QA1411200); and the National Natural Science Foundation of China (no. 22271306). J.T. acknowledges the Marie-Curie Fellowship. I.M. acknowledges the Canada 150 Research Chair and NSERC Discovery Grant. R.Y. acknowledges the funding support from Young Scientists Fund of the National Natural Science Foundation of China (grant no. 21905240); the Shenzhen Research Institute, City University of Hong Kong; the State Key Laboratory of Marine Pollution (SKLMP) Seed Collaborative Research Fund; the Guangdong Basic and Applied Basic Research Fund (2022A1515011333); the Shenzhen Science and Technology Program (JCYJ20220818101204009); Hong Kong Research Grant Council (21300620) and the State Key Laboratory of Marine Pollution Internal Research Fund (SKLMP/IRF/0029). D.L.P. and L.D. thank the University of Hong Kong Development Fund 2013-2014 project ‘New Ultrafast Spectroscopy Experiments for Shared Facilities’. We thank the Shanghai Synchrotron Radiation Facility for providing the BL16B1 and BL10U1 beamline for collecting the synchrotron X-ray scattering data. We acknowledge access and support of the GW4 Facility for High-Resolution Electron Cryo-Microscopy, funded by the Wellcome Trust (202904/Z/16/Z and 206181/Z/17/Z) and BBSRC (BB/R000484/1). We thank Y. Zhang and X. Jin for the useful discussions. We thank M. Sener and D. H. Fackler for permission to use the visual molecular dynamics model in Fig. 1c.
Author information
Authors and Affiliations
Contributions
J.T. conceived, designed and supervised the research. J.T., J.Y., Q.T., S.-B.Y. and R.Y. carried out most of the experiments. M.Z., L.H., J.S., Y.S., J.Z., D.M., Y.L., Q.-Y.Q., F.T. and L.Z. conducted part of the experiments. J.-C.E. performed the TEM, STEM and EDX imaging. R.L.H performed the AFM analysis. U.B. performed the cryo-TEM imaging. L.D. and D.L.P. performed the fs-TA studies. J.T., L.D., D.L.P. and R.Y. analysed the data and wrote the manuscript with input from the other authors. I.M. provided useful inputs and comments on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–83, Tables 1–8 and References.
Supplementary Data 1
Atomic coordinates of optimized computational model of CO-CoN4.
Supplementary Data 2
Atomic coordinates of optimized computational model of ZnN4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, J., Huang, L., Tang, Q. et al. Artificial spherical chromatophore nanomicelles for selective CO2 reduction in water. Nat Catal 6, 464–475 (2023). https://doi.org/10.1038/s41929-023-00962-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00962-z