Abstract
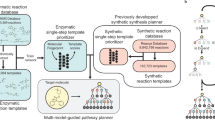
Retrobiosynthesis provides an effective and sustainable approach to producing functional molecules. The past few decades have witnessed a rapid expansion of biosynthetic approaches. With the recent advances in data-driven sciences, machine learning (ML) is enriching the retrobiosynthesis design toolbox and being applied to each step of the synthesis design workflow, including retrosynthesis planning, enzyme identification and engineering, and pathway optimization. The ability to learn from existing knowledge, recognize complex patterns and generalize to the unknown has made ML a promising solution to biological problems. In this Review, we summarize the recent progress in the development of ML models for assisting with molecular synthesis. We highlight the key advantages of ML-based biosynthesis design methods and discuss the challenges and outlook for the further development of ML-based approaches.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lin, G.-M., Warden-Rothman, R. & Voigt, C. A. Retrosynthetic design of metabolic pathways to chemicals not found in nature. Curr. Opin. Syst. Biol. 14, 82–107 (2019).
Bornscheuer, U. T. et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012).
Sheldon, R. A. & Woodley, J. M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 118, 801–838 (2018).
de Souza, R. O. M. A., Miranda, L. S. M. & Bornscheuer, U. T. A retrosynthesis approach for biocatalysis in organic synthesis. Chem. Eur. J. 23, 12040–12063 (2017).
Turner, N. J. & O’Reilly, E. Biocatalytic retrosynthesis. Nat. Chem. Biol. 9, 285–288 (2013).
The UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515 (2019).
Khan, A. Z., Bilal, M., Rasheed, T. & Iqbal, H. M. N. Advancements in biocatalysis: from computational to metabolic engineering. Chin. J. Catal. 39, 1861–1868 (2018).
Feehan, R., Montezano, D. & Slusky, J. S. G. Machine learning for enzyme engineering, selection and design. Protein Eng. Des. Sel. 34, gzab019 (2021).
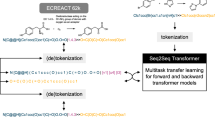
Probst, D. et al. Biocatalysed synthesis planning using data-driven learning. Nat. Commun. 13, 964 (2022). This paper describes the development of a template-free retrobiosynthesis tool by training a molecular transformer with multi-task transfer learning using both enzymatic and chemical reaction databases.
Hie, B. L. & Yang, K. K. Adaptive machine learning for protein engineering. Curr. Opin. Struct. Biol. 72, 145–152 (2022).
Yang, K. K., Wu, Z. & Arnold, F. H. Machine-learning-guided directed evolution for protein engineering. Nat. Methods 16, 687–694 (2019).
Rives, A. et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proc. Natl Acad. Sci. USA 118, e2016239118 (2021).
Alley, E. C., Khimulya, G., Biswas, S., AlQuraishi, M. & Church, G. M. Unified rational protein engineering with sequence-based deep representation learning. Nat. Methods 16, 1315–1322 (2019).
Wang, L., Dash, S., Ng, C. Y. & Maranas, C. D. A review of computational tools for design and reconstruction of metabolic pathways. Synth. Syst. Biotechnol. 2, 243–252 (2017).
Koch, M., Duigou, T. & Faulon, J.-L. Reinforcement learning for bioretrosynthesis. ACS Synth. Biol. 9, 157–168 (2020).
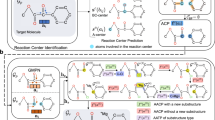
Zheng, S. et al. Deep learning driven biosynthetic pathways navigation for natural products with BioNavi-NP. Nat. Commun. 13, 3342 (2022). This paper introduces a useful retrobiosynthesis tool for navigating biosynthetic pathways to complex natural products from simple building blocks.
Fuji, T., Nakazawa, S. & Ito, K. Feasible-metabolic-pathway-exploration technique using chemical latent space. Bioinformatics 36, i770–i778 (2020).
Finnigan, W., Hepworth, L. J., Flitsch, S. L. & Turner, N. J. RetroBioCat as a computer-aided synthesis planning tool for biocatalytic reactions and cascades. Nat. Catal. 4, 98–104 (2021).
Kumar, A., Wang, L., Ng, C. Y. & Maranas, C. D. Pathway design using de novo steps through uncharted biochemical spaces. Nat. Commun. 9, 184 (2018).
Delépine, B. et al. RetroPath2.0: a retrosynthesis workflow for metabolic engineers. Metab. Eng. 45, 158–170 (2018).
Kim, Y., Ryu, J. Y., Kim, H. U., Jang, W. D. & Lee, S. Y. A deep learning approach to evaluate the feasibility of enzymatic reactions generated by retrobiosynthesis. Biotechnol. J. 16, 2000605 (2021).
Weininger, D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Model. 28, 31–36 (1988).
Pesciullesi, G., Schwaller, P., Laino, T. & Reymond, J.-L. Transfer learning enables the molecular transformer to predict regio- and stereoselective reactions on carbohydrates. Nat. Commun. 11, 4874 (2020).
Hasic, H. & Ishida, T. Single-step retrosynthesis prediction based on the identification of potential disconnection sites using molecular substructure fingerprints. J. Chem. Inf. Model. 61, 641–652 (2021).
Somnath, V. R., Bunne, C., Coley, C., Krause, A. & Barzilay, R. Learning graph models for retrosynthesis prediction. In Proc. 34th Advances in Neural Information Processing Systems (eds Ranzato, M. et al.) 9405–9415 (Curran Associates, Inc., 2021).
Wang, H. et al. Chemical-reaction-aware molecule representation learning. Preprint at https://arxiv.org/abs/2109.09888 (2021).
Gómez-Bombarelli, R. et al. Automatic chemical design using a data-driven continuous representation of molecules. ACS Cent. Sci. 4, 268–276 (2018).
Tempke, R. & Musho, T. Autonomous design of new chemical reactions using a variational autoencoder. Commun. Chem. 5, 40 (2022).
Zheng, S., Rao, J., Zhang, Z., Xu, J. & Yang, Y. Predicting retrosynthetic reactions using self-corrected transformer neural networks. J. Chem. Inf. Model. 60, 47–55 (2020).
Coley, C. W., Rogers, L., Green, W. H. & Jensen, K. F. SCScore: synthetic complexity learned from a reaction corpus. J. Chem. Inf. Model. 58, 252–261 (2018).
Segler, M. H. S. & Waller, M. P. Neural-symbolic machine learning for retrosynthesis and reaction prediction. Chemistry 23, 5966–5971 (2017).
Schwaller, P. et al. Predicting retrosynthetic pathways using transformer-based models and a hyper-graph exploration strategy. Chem. Sci. 11, 3316–3325 (2020).
Segler, M. H. S., Preuss, M. & Waller, M. P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555, 604–610 (2018).
Chen, B., Li, C., Dai, H. & Song, L. Retro*: learning retrosynthetic planning with neural guided A* search. In Proc. 37th International Conference on Machine Learning (eds Daumé, H. III & Singh, A) 1608–1616 (PMLR, 2020).
Krogh, A., Brown, M., Mian, I. S., Sjölander, K. & Haussler, D. Hidden Markov models in computational biology: applications to protein modeling. J. Mol. Biol. 235, 1501–1531 (1994).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Ryu, J. Y., Kim, H. U. & Lee, S. Y. Deep learning enables high-quality and high-throughput prediction of enzyme commission numbers. Proc. Natl Acad. Sci. USA 116, 13996–14001 (2019).
Price, M. N. et al. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature 557, 503–509 (2018).
Nallapareddy, V. et al. CATHe: detection of remote homologues for CATH superfamilies using embeddings from protein language models. Bioinformatics 39, btad029 (2022).
Sanderson, T., Bileschi, M. L., Belanger, D. & Colwell, L. J. ProteInfer: deep networks for protein functional inference. Preprint at bioRxiv https://doi.org/10.1101/2021.09.20.461077 (2021). This paper reports a state-of-the-art ML-based protein annotation tool capable of predicting both EC number and Gene Ontology (GO) from amino acid sequences.
Heinzinger, M. et al. Contrastive learning on protein embeddings enlightens midnight zone. NAR Genom. Bioinform. 4, lqac043 (2022).
Carbonell, P. et al. Selenzyme: enzyme selection tool for pathway design. Bioinformatics 34, 2153–2154 (2018).
Cho, A., Yun, H., Park, J. H., Lee, S. Y. & Park, S. Prediction of novel synthetic pathways for the production of desired chemicals. BMC Syst. Biol. 4, 35 (2010).
Visani, G. M., Hughes, M. C. & Hassoun, S. Enzyme promiscuity prediction using hierarchy-informed multi-label classification. Bioinformatics 37, 2017–2024 (2021).
Goldman, S., Das, R., Yang, K. K. & Coley, C. W. Machine learning modeling of family wide enzyme–substrate specificity screens. PLoS Comput. Biol. 18, e1009853 (2022).
Xu, Z., Wu, J., Song, Y. S. & Mahadevan, R. Enzyme activity prediction of sequence variants on novel substrates using improved substrate encodings and convolutional pooling. In Proc. 16th Machine Learning in Computational Biology meeting (eds Knowles, D. A. et al) 78–87 (PMLR, 2022).
Musil, M., Konegger, H., Hon, J., Bednar, D. & Damborsky, J. Computational design of stable and soluble biocatalysts. ACS Catal. 9, 1033–1054 (2019).
Hon, J. et al. SoluProt: prediction of soluble protein expression in Escherichia coli. Bioinformatics 37, 23–28 (2021).
Li, G., Rabe, K. S., Nielsen, J. & Engqvist, M. K. M. Machine learning applied to predicting microorganism growth temperatures and enzyme catalytic optima. ACS Synth. Biol. 8, 1411–1420 (2019).
Almagro Armenteros, J. J., Sønderby, C. K., Sønderby, S. K., Nielsen, H. & Winther, O. DeepLoc: prediction of protein subcellular localization using deep learning. Bioinformatics 33, 3387–3395 (2017).
Stärk, H., Dallago, C., Heinzinger, M. & Rost, B. Light attention predicts protein location from the language of life. Bioinform. Adv. 1, vbab035 (2021).
Chai, M. et al. Application of machine learning algorithms to estimate enzyme loading, immobilization yield, activity retention, and reusability of enzyme–metal–organic framework biocatalysts. Chem. Mater. 33, 8666–8676 (2021).
Li, F. et al. Deep learning-based kcat prediction enables improved enzyme-constrained model reconstruction. Nat. Catal. 5, 662–672 (2022).
Dhakal, A., McKay, C., Tanner, J. J. & Cheng, J. Artificial intelligence in the prediction of protein–ligand interactions: recent advances and future directions. Brief. Bioinform. 23, bbab476 (2022).
Wang, Y. et al. Directed evolution: methodologies and applications. Chem. Rev. 121, 12384–12444 (2021).
Wittmann, B. J., Johnston, K. E., Wu, Z. & Arnold, F. H. Advances in machine learning for directed evolution. Curr. Opin. Struct. Biol. 69, 11–18 (2021).
Luo, Y. et al. ECNet is an evolutionary context-integrated deep learning framework for protein engineering. Nat. Commun. 12, 5743 (2021).
Biswas, S., Khimulya, G., Alley, E. C., Esvelt, K. M. & Church, G. M. Low-N protein engineering with data-efficient deep learning. Nat. Methods 18, 389–396 (2021).
Hsu, C. et al. Learning protein fitness models from evolutionary and assay-labeled data. Nat. Biotechnol. 40, 1114–1122 (2022). This paper reports an in depth evaluation and discussion of ML models predicting variant effects under a low-N situation.
Wittmann, B. J., Yue, Y. & Arnold, F. H. Informed training set design enables efficient machine learning-assisted directed protein evolution. Cell Syst. 12, 1026–1045.e7 (2021).
Greenhalgh, J. C., Fahlberg, S. A., Pfleger, B. F. & Romero, P. A. Machine learning-guided acyl-ACP reductase engineering for improved in vivo fatty alcohol production. Nat. Commun. 12, 5825 (2021).
Hawkins-Hooker, A. et al. Generating functional protein variants with variational autoencoders. PLoS Comput. Biol. 17, e1008736 (2021).
Repecka, D. et al. Expanding functional protein sequence spaces using generative adversarial networks. Nat. Mach. Intell. 3, 324–333 (2021).
Chan, A., Madani, A., Krause, B. & Naik, N. Deep extrapolation for attribute-enhanced generation. In Proc. 34th Advances in Neural Information Processing Systems (eds Ranzato, M. et al.) 14084–14096 (Curran Associates, Inc., 2021).
Schmitt, L. et al. Prediction of designer-recombinases for DNA editing with generative deep learning. Nat. Commun. 13, 7966 (2022).
Madani, A. et al. Deep neural language modeling enables functional protein generation across families. Preprint at bioRxiv https://doi.org/10.1101/2021.07.18.452833 (2021). Using a global language model trained on protein sequences and annotations, the authors demonstrate a universal generative model capable of generating protein sequences with desired properties with a varying degree of sequence similarity.
Ferruz, N., Schmidt, S. & Höcker, B. ProtGPT2 is a deep unsupervised language model for protein design. Nat. Commun. 13, 4348 (2022).
Anand, N. et al. Protein sequence design with a learned potential. Nat. Commun. 13, 746 (2022).
Ingraham, J., Garg, V., Barzilay, R. & Jaakkola, T. Generative models for graph-based protein design. In Proc. 32nd Advances in Neural Information Processing Systems (eds Wallach, H. et al.) (Curran Associates, Inc., 2019).
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021).
Anishchenko, I. et al. De novo protein design by deep network hallucination. Nature 600, 547–552 (2021).
Wang, J. et al. Scaffolding protein functional sites using deep learning. Science 377, 387–394 (2022).
Wu, Z., Johnston, K. E., Arnold, F. H. & Yang, K. K. Protein sequence design with deep generative models. Curr. Opin. Chem. Biol. 65, 18–27 (2021).
Strokach, A. & Kim, P. M. Deep generative modeling for protein design. Curr. Opin. Struct. Biol. 72, 226–236 (2022). An insightful review of various deep learning approaches to protein design.
Liew, F. E. et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat. Biotechnol. 40, 335–344 (2022). This paper describes the in vitro machine learning screening method iPROBE used to engineer Clostridium autoethanogenum for the overproduction of acetone and isopropanol at the industrial scale.
Sun, X., Xu, Y. & Huang, H. Thraustochytrid cell factories for producing lipid compounds. Trends Biotechnol. 39, 648–650 (2021).
Antonakoudis, A., Barbosa, R., Kotidis, P. & Kontoravdi, C. The era of big data: genome-scale modelling meets machine learning. Comput. Struct. Biotechnol. J. 18, 3287–3300 (2020).
Kim, Y., Kim, G. B. & Lee, S. Y. Machine learning applications in genome-scale metabolic modeling. Curr. Opin. Syst. Biol. 25, 42–49 (2021).
Zampieri, G., Vijayakumar, S., Yaneske, E. & Angione, C. Machine and deep learning meet genome-scale metabolic modeling. PLoS Comput. Biol. 15, e1007084 (2019).
Heckmann, D. et al. Machine learning applied to enzyme turnover numbers reveals protein structural correlates and improves metabolic models. Nat. Commun. 9, 5252 (2018).
Heckmann, D. et al. Kinetic profiling of metabolic specialists demonstrates stability and consistency of in vivo enzyme turnover numbers. Proc. Natl Acad. Sci. USA 117, 23182–23190 (2020). This paper reports fluxomic and proteomic data for estimating in vivo kcat values that were used to parameterize a metabolic model that could then be used for more accurate gene expression prediction.
Shah, H. A., Liu, J., Yang, Z. & Feng, J. Review of machine learning methods for the prediction and reconstruction of metabolic pathways. Front. Mol. Biosci. 8, 634141 (2021).
Fang, X., Lloyd, C. J. & Palsson, B. O. Reconstructing organisms in silico: genome-scale models and their emerging applications. Nat. Rev. Microbiol. 18, 731–743 (2020).
HamediRad, M. et al. Towards a fully automated algorithm driven platform for biosystems design. Nat. Commun. 10, 5150 (2019).
Radivojević, T., Costello, Z., Workman, K. & Garcia Martin, H. A machine learning automated recommendation tool for synthetic biology. Nat. Commun. 11, 4879 (2020).
Zhang, J. et al. Combining mechanistic and machine learning models for predictive engineering and optimization of tryptophan metabolism. Nat. Commun. 11, 4880 (2020).
Zhou, Y. et al. MiYA, an efficient machine-learning workflow in conjunction with the YeastFab assembly strategy for combinatorial optimization of heterologous metabolic pathways in Saccharomyces cerevisiae. Metab. Eng. 47, 294–302 (2018).
Opgenorth, P. et al. Lessons from two design–build–test–learn cycles of dodecanol production in Escherichia coli aided by machine learning. ACS Synth. Biol. 8, 1337–1351 (2019).
Jervis, A. J. et al. Machine learning of designed translational control allows predictive pathway optimization in Escherichia coli. ACS Synth. Biol. 8, 127–136 (2019).
Karim, A. S. et al. In vitro prototyping and rapid optimization of biosynthetic enzymes for cell design. Nat. Chem. Biol. 16, 912–919 (2020).
Huffman, M. A. et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 366, 1255–1259 (2019).
Peters, R. J. R. W. et al. Cascade reactions in multicompartmentalized polymersomes. Angew. Chem. Int. Ed. 126, 150–154 (2014).
Nobeli, I., Favia, A. D. & Thornton, J. M. Protein promiscuity and its implications for biotechnology. Nat. Biotechnol. 27, 157–167 (2009).
Wan, Z., Wang, Q.-D., Liu, D. & Liang, J. Accelerating the optimization of enzyme-catalyzed synthesis conditions via machine learning and reactivity descriptors. Org. Biomol. Chem. 19, 6267–6273 (2021).
Coley, C. W., Green, W. H. & Jensen, K. F. Machine learning in computer-aided synthesis planning. Acc. Chem. Res. 51, 1281–1289 (2018).
Gao, H. et al. Using machine learning to predict suitable conditions for organic reactions. ACS Cent. Sci. 4, 1465–1476 (2018).
Gainza, P. et al. Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods 17, 184–192 (2020).
Morselli Gysi, D. et al. Network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proc. Natl Acad. Sci. USA 118, e2025581118 (2021).
Mayr, A., Klambauer, G., Unterthiner, T. & Hochreiter, S. DeepTox: toxicity prediction using deep learning. Front. Environ. Sci. 3, 80 (2016).
Gasteiger, E. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784–3788 (2003).
Wang, C. Y. et al. ProtaBank: a repository for protein design and engineering data. Protein Sci. 27, 1113–1124 (2018).
Pezeshgi Modarres, H., Mofrad, M. R. & Sanati-Nezhad, A. ProtDataTherm: a database for thermostability analysis and engineering of proteins. PLoS ONE 13, e0191222 (2018).
Stourac, J. et al. FireProtDB: database of manually curated protein stability data. Nucleic Acids Res. 49, D319–D324 (2021).
Hastings, J. et al. ChEBI in 2016: improved services and an expanding collection of metabolites. Nucleic Acids Res. 44, D1214–D1219 (2016).
Wishart, D. S. et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 46, D608–D617 (2018).
Sud, M. et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 35, D527–D532 (2007).
Aimo, L. et al. The SwissLipids knowledgebase for lipid biology. Bioinformatics 31, 2860–2866 (2015).
Jeske, L., Placzek, S., Schomburg, I., Chang, A. & Schomburg, D. BRENDA in 2019: a European ELIXIR core data resource. Nucleic Acids Res. 47, D542–D549 (2018).
Lombardot, T. et al. Updates in Rhea: SPARQLing biochemical reaction data. Nucleic Acids Res. 47, D596–D600 (2019).
Buchholz, P. C. F. et al. BioCatNet: a database system for the integration of enzyme sequences and biocatalytic experiments. ChemBioChem 17, 2093–2098 (2016).
Wittig, U., Rey, M., Weidemann, A., Kania, R. & Müller, W. SABIO-RK: an updated resource for manually curated biochemical reaction kinetics. Nucleic Acids Res. 46, D656–D660 (2018).
Lang, M., Stelzer, M. & Schomburg, D. BKM-react, an integrated biochemical reaction database. BMC Biochem. 12, 42 (2011).
Kanehisa, M. et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36, D480–D484 (2007).
Wicker, J. et al. enviPath—the environmental contaminant biotransformation pathway resource. Nucleic Acids Res. 44, D502–D508 (2016).
King, Z. A. et al. BiGG models: a platform for integrating, standardizing and sharing genome-scale models. Nucleic Acids Res. 44, D515–D522 (2016).
Gillespie, M. et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 50, D687–D692 (2022).
Wishart, D. S. et al. PathBank: a comprehensive pathway database for model organisms. Nucleic Acids Res. 48, D470–D478 (2020).
Caspi, R. et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 46, D633–D639 (2017).
Moretti, S., Tran, V. D. T., Mehl, F., Ibberson, M. & Pagni, M. MetaNetX/MNXref: unified namespace for metabolites and biochemical reactions in the context of metabolic models. Nucleic Acids Res. 49, D570–D574 (2021).
Mazurenko, S., Prokop, Z. & Damborsky, J. Machine learning in enzyme engineering. ACS Catal. 10, 1210–1223 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Torng, W. & Altman, R. B. High precision protein functional site detection using 3D convolutional neural networks. Bioinformatics 35, 1503–1512 (2019).
Dallago, C. et al. FLIP: benchmark tasks in fitness landscape inference for proteins. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/2021.11.09.467890v2 (2022).
Hulo, N. The PROSITE database. Nucleic Acids Res. 34, D227–D230 (2006).
Berman, H. M. The Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
Cui, Y., Dong, Q., Hong, D. & Wang, X. Predicting protein–ligand binding residues with deep convolutional neural networks. BMC Bioinform. 20, 93 (2019).
Xia, C.-Q., Pan, X. & Shen, H.-B. Protein–ligand binding residue prediction enhancement through hybrid deep heterogeneous learning of sequence and structure data. Bioinformatics 36, 3018–3027 (2020).
Stepniewska-Dziubinska, M. M., Zielenkiewicz, P. & Siedlecki, P. Improving detection of protein–ligand binding sites with 3D segmentation. Sci. Rep. 10, 5035 (2020).
Mylonas, S. K., Axenopoulos, A. & Daras, P. DeepSurf: a surface-based deep learning approach for the prediction of ligand binding sites on proteins. Bioinformatics 37, 1681–1690 (2021).
Kandel, J., Tayara, H. & Chong, K. T. PUResNet: prediction of protein–ligand binding sites using deep residual neural network. J. Cheminform. 13, 65 (2021).
Kroll, A., Engqvist, M. K. M., Heckmann, D. & Lercher, M. J. Deep learning allows genome-scale prediction of Michaelis constants from structural features. PLoS Biol. 19, e3001402 (2021).
Kavvas, E. S., Yang, L., Monk, J. M., Heckmann, D. & Palsson, B. O. A biochemically interpretable machine learning classifier for microbial GWAS. Nat. Commun. 11, 2580 (2020).
Ajjolli Nagaraja, A. et al. A machine learning approach for efficient selection of enzyme concentrations and its application for flux optimization. Catalysts 10, 291 (2020).
Caschera, F. et al. Coping with complexity: machine learning optimization of cell-free protein synthesis. Biotechnol. Bioeng. 108, 2218–2228 (2021).
Acknowledgements
This work was supported by the Molecule Maker Lab Institute, an AI Research Institutes programme supported by the US National Science Foundation under grant no. 2019897 (H.Z.). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect those of the NSF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks William Finnigan, Pablo Carbonell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, T., Boob, A.G., Volk, M.J. et al. Machine learning-enabled retrobiosynthesis of molecules. Nat Catal 6, 137–151 (2023). https://doi.org/10.1038/s41929-022-00909-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00909-w
This article is cited by
-
Iterative design of training data to control intricate enzymatic reaction networks
Nature Communications (2024)