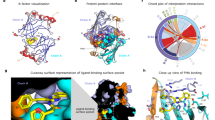
Abstract
The widely used coelenterazine-powered Renilla luciferase was discovered over 40 years ago, but the oxidative mechanism by which it generates blue photons remains unclear. Here we decipher Renilla-type catalysis through crystallographic, spectroscopic and computational experiments. Structures of ancestral and extant luciferases complexed with the substrate-like analogue azacoelenterazine or a reaction product were obtained, providing molecular snapshots of coelenterazine-to-coelenteramide oxidation. Bound coelenterazine adopts a Y-shaped conformation, enabling the deprotonated imidazopyrazinone component to attack O2 via a radical charge-transfer mechanism. A high emission intensity is secured by an aspartate from a conserved proton-relay system, which protonates the excited coelenteramide product. Another aspartate on the rim of the catalytic pocket fine-tunes the electronic state of coelenteramide and promotes the formation of the blue light-emitting phenolate anion. The results obtained also reveal structural features distinguishing flash-type from glow-type bioluminescence, providing insights that will guide the engineering of next-generation luciferase‒luciferin pairs for ultrasensitive optical bioassays.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Atomic coordinates and structural factors have been deposited in the PDB (www.wwpdb.org)70 under accession codes 7QXR, 7QXQ, 7OMD, 7OMR and 7OMO. We will release the atomic coordinates and experimental data on article publication. The primary data from molecular dynamics simulations are available in Zenodo repository with the identifier https://doi.org/10.5281/zenodo.7241544. Source data are provided with this paper.
References
Mitiouchkina, T. et al. Plants with genetically encoded autoluminescence. Nat. Biotechnol. 38, 944–946 (2020).
Bueso, Y. F. & Tangney, M. Synthetic biology in the driving seat of the bioeconomy. Trends Biotechnol. 35, 373–378 (2017).
Xu, T. et al. The expanding toolbox of in vivo bioluminescent imaging. Front. Oncol. 6, 150 (2016).
Syed, A. J. & Anderson, J. C. Applications of bioluminescence in biotechnology and beyond. Chem. Soc. Rev. 50, 5668–5705 (2021).
Fleiss, A. & Sarkisyan, K. S. A brief review of bioluminescent systems (2019). Curr. Genet. 65, 877–882 (2019).
Nicol, J. A. C. Observations on luminescence in Renilla (Pennatulacea). J. Exp. Biol. 32, 299–320 (1955).
Matthews, J. C., Hori, K. & Cormier, M. J. Purification and properties of Renilla reniformis luciferase. Biochemistry 16, 85–91 (1977).
Lorenz, W. W., McCann, R. O., Longiaru, M. & Cormier, M. J. Isolation and expression of a cDNA encoding Renilla reniformis luciferase. Proc. Natl Acad. Sci. USA 88, 4438–4442 (1991).
Delroisse, J., Duchatelet, L., Flammang, P. & Mallefet, J. Leaving the dark side? Insights into the evolution of Luciferases. Front. Mar. Sci. 8, 690 (2021).
Delroisse, J. et al. A puzzling homology: a brittle star using a putative cnidarian-type luciferase for bioluminescence. Open Biol. 7, 160300 (2017).
Chaloupkova, R. et al. Light-emitting dehalogenases: reconstruction of multifunctional biocatalysts. ACS Catal. 9, 4810–4823 (2019).
Janssen, D. B. Evolving haloalkane dehalogenases. Curr. Opin. Chem. Biol. 8, 150–159 (2004).
Schenkmayerova, A. et al. Engineering the protein dynamics of an ancestral luciferase. Nat. Commun. 12, 3616 (2021).
Loening, A. M., Fenn, T. D. & Gambhir, S. S. Crystal structures of the luciferase and green fluorescent protein from Renilla reniformis. J. Mol. Biol. 374, 1017–1028 (2007).
Loening, A. M., Wu, A. M. & Gambhir, S. S. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat. Methods 4, 641–643 (2007).
McCapra, F. & Chang, Y. C. The chemiluminescence of a Cypridina luciferin analogue. Chem. Commun. Lond. https://doi.org/10.1039/C19670001011 (1967).
Goto, T. Chemistry of bioluminescence. Pure Appl. Chem. 17, 421–442 (1968).
Campbell, A. K. & Herring, P. J. Imidazolopyrazine bioluminescence in copepods and other marine organisms. Mar. Biol. 104, 219–225 (1990).
Haddock, S. H. D., Moline, M. A. & Case, J. F. Bioluminescence in the sea. Annu. Rev. Mar. Sci. 2, 443–493 (2010).
Shimomura, O., Masugi, T., Johnson, F. H. & Haneda, Y. Properties and reaction mechanism of the bioluminescence system of the deep-sea shrimp Oplophorus gracilorostris. Biochemistry 17, 994–998 (1978).
Ohmiya, Y. & Hirano, T. Shining the light: the mechanism of the bioluminescence reaction of calcium-binding photoproteins. Chem. Biol. 3, 337–347 (1996).
Inouye, S., Sahara-Miura, Y., Nakamura, M. & Hosoya, T. Expression, purification, and characterization of recombinant apoPholasin. Protein Expr. Purif. 171, 105615 (2020).
Griffiths, T. M., Oakley, A. J. & Yu, H. Atomistic insights into photoprotein formation: computational prediction of the properties of coelenterazine and oxygen binding in obelin. J. Comput. Chem. 41, 587–603 (2020).
Branchini, B. R. et al. Experimental support for a single electron-transfer oxidation mechanism in firefly bioluminescence. J. Am. Chem. Soc. 137, 7592–7595 (2015).
Rahnama, S., Saffar, B., Kahrani, Z. F., Nazari, M. & Emamzadeh, R. Super RLuc8: a novel engineered Renilla luciferase with a red-shifted spectrum and stable light emission. Enzym. Microb. Technol. 96, 60–66 (2017).
Loening, A. M., Fenn, T. D., Wu, A. M. & Gambhir, S. S. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng. Des. Sel. PEDS 19, 391–400 (2006).
Vedadi, M. et al. Chemical screening methods to identify ligands that promote protein stability, protein crystallization, and structure determination. Proc. Natl Acad. Sci. USA 103, 15835–15840 (2006).
Deller, M. C., Kong, L. & Rupp, B. Protein stability: a crystallographer’s perspective. Acta Crystallogr. Sect. F. Struct. Biol. Commun. 72, 72–95 (2016).
Doerr, A. Widening the protein crystallization bottleneck. Nat. Methods 3, 961–961 (2006).
Gumulya, Y. et al. Engineering highly functional thermostable proteins using ancestral sequence reconstruction. Nat. Catal. 1, 878–888 (2018).
Schriever, K. et al. Engineering of ancestors as a tool to elucidate structure, mechanism, and specificity of extant terpene cyclase. J. Am. Chem. Soc. 143, 3794–3807 (2021).
Perez-Garcia, P. et al. A promiscuous ancestral enzyme’s structure unveils protein variable regions of the highly diverse metallo-β-lactamase family. Commun. Biol. 4, 132 (2021).
Castro-Fernandez, V. et al. Reconstructed ancestral enzymes reveal that negative selection drove the evolution of substrate specificity in ADP-dependent kinases. J. Biol. Chem. 292, 15598–15610 (2017).
Muñoz, S. M., Castro-Fernandez, V. & Guixé, V. Structure of an ancestral ADP-dependent kinase with fructose-6P reveals key residues for binding, catalysis, and ligand-induced conformational changes. J. Biol. Chem. 296, 100219 (2021).
Nicoll, C. R. et al. Ancestral-sequence reconstruction unveils the structural basis of function in mammalian FMOs. Nat. Struct. Mol. Biol. 27, 14–24 (2020).
Coutant, E. P. et al. Gram-scale synthesis of luciferins derived from coelenterazine and original insights into their bioluminescence properties. Org. Biomol. Chem. 17, 3709–3713 (2019).
Verschueren, K. H. G., Seljée, F., Rozeboom, H. J., Kalk, K. H. & Dijkstra, B. W. Crystallographic analysis of the catalytic mechanism of haloalkane dehalogenase. Nature 363, 693–698 (1993).
Shigehisa, M. et al. Stabilization of luciferase from Renilla reniformis using random mutations. Protein Eng. Des. Sel. 30, 7–13 (2017).
Woo, J. & von Arnim, A. G. Mutational optimization of the coelenterazine-dependent luciferase from Renilla. Plant Methods 4, 23 (2008).
Bui, S. & Steiner, R. A. New insight into cofactor-free oxygenation from combined experimental and computational approaches. Curr. Opin. Struct. Biol. 41, 109–118 (2016).
Steiner, R. A., Janssen, H. J., Roversi, P., Oakley, A. J. & Fetzner, S. Structural basis for cofactor-independent dioxygenation of N-heteroaromatic compounds at the alpha/beta-hydrolase fold. Proc. Natl Acad. Sci. USA 107, 657–662 (2010).
Buettner, G. R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 3, 259–303 (1987).
Lupidi, G. et al. Synthesis, structural and spectroscopic characterization and biomimetic properties of new copper, manganese, zinc complexes: identification of possible superoxide-dismutase mimics bearing hydroxyl radical generating/scavenging abilities. J. Inorg. Biochem. 104, 820–830 (2010).
Gao, Z., Yang, X., Huang, K. & Xu, H. Free-radical scavenging and mechanism study of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Appl. Magn. Reson. 19, 35–44 (2000).
Ueno, I., Kohno, M., Haraikawa, K. & Hirono, I. Interaction between quercetin and superoxide radicals. Reduction of the quercetin mutagenicity. J. Pharmacobiodyn. 7, 798–803 (1984).
Finkelstein, E., Rosen, G. M. & Rauckman, E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch. Biochem. Biophys. 200, 1–16 (1980).
Esnouf, M. P., Gainey, A. I., Hill, H. A. O. & Thornalley, P. J. in Inflammatory Diseases and Copper: The Metabolic and Therapeutic Roles of Copper and Other Essential Metalloelements in Humans (ed. Sorenson, J. R. J.) 209–220 (Humana Press, 1982). https://doi.org/10.1007/978-1-4612-5829-2_20
Makino, K. et al. An artifact in the ESR spectrum obtained by spin trapping with DMPO. Free Radic. Res. Commun. 6, 19–28 (1989).
Fujimori, K. et al. Chemiluminescence of Cypridina luciferin analogues. Part 1. Effect of pH on rates of spontaneous autoxidation of CLA in aqueous buffer solutions. J. Chem. Soc. Perkin Trans. 2, 2405–2409 (1993).
Magalhães, C. M., Esteves da Silva, J. C. G. & Pinto da Silva, L. Comparative study of the chemiluminescence of coelenterazine, coelenterazine-e and Cypridina luciferin with an experimental and theoretical approach. J. Photochem. Photobiol., B. 190, 21–31 (2019).
Lourenço, J. M., Esteves da Silva, J. C. G. & Pinto da Silva, L. Combined experimental and theoretical study of Coelenterazine chemiluminescence in aqueous solution. J. Lumin. 194, 139–145 (2018).
Hori, K., Wampler, J. E., Matthews, J. C. & Cormier, M. J. Bioluminescence of Renilla reniformis. XIII. Identification of the product excited states during the chemiluminescent and bioluminescent oxidation of Renilla (sea pansy) luciferin and certain of its analogs. Biochemistry 12, 4463–4468 (1973).
Imai, Y. et al. Fluorescence properties of phenolate anions of coelenteramide analogues: the light-emitter structure in aequorin bioluminescence. J. Photochem. Photobiol. Chem. 146, 95–107 (2001).
Li, Z.-S., Zhao, X., Zou, L.-Y. & Ren, A.-M. The dynamics simulation and quantum calculation investigation about luminescence mechanism of coelenteramide. Photochem. Photobiol. 89, 849–855 (2013).
Liu, Z.-J. et al. Crystal structure of obelin after Ca2+-triggered bioluminescence suggests neutral coelenteramide as the primary excited state. Proc. Natl Acad. Sci. USA 103, 2570–2575 (2006).
Inouye, S. et al. Chiral deaza-coelenterazine analogs for probing a substrate-binding site in the Ca2+-binding photoprotein aequorin. PLoS ONE 16, e0251743 (2021).
Tessler, M. et al. A putative chordate luciferase from a cosmopolitan tunicate indicates convergent bioluminescence evolution across phyla. Sci. Rep. 10, 17724 (2020).
Delroisse, J. et al. Fine structure of the luminous spines and luciferase detection in the brittle star Amphiura filiformis. Zool. Anz. 269, 1–12 (2017).
Stepanyuk, G. A. et al. Structure based mechanism of the Ca(2+)-induced release of coelenterazine from the Renilla binding protein. Proteins 74, 583–593 (2009).
Titushin, M. S., Feng, Y., Lee, J., Vysotski, E. S. & Liu, Z.-J. Protein-protein complexation in bioluminescence. Protein Cell 2, 957–972 (2011).
Ward, W. W. & Cormier, M. J. Energy transfer via protein-protein interaction in Renilla bioluminescence. Photochem. Photobiol. 27, 389–396 (1978).
Kabsch, W. XDS. Acta Crystallogr. D. Biol. Crystallogr. 66, 125–132 (2010).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D. Biol. Crystallogr. 69, 1204–1214 (2013).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D. Biol. Crystallogr. 68, 352–367 (2012).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. Publ. Protein Soc. 27, 293–315 (2018).
PyMOL. PyMOL Molecular Graphics System v. 2.0 (Schrödinger LLC).
wwPDB consortium. Protein Data Bank: the single global archive for 3D macromolecular structure data. Nucleic Acids Res. 47, D520–D528 (2019).
Schomburg, K. T., Wetzer, L. & Rarey, M. Interactive design of generic chemical patterns. Drug Discov. Today 18, 651–658 (2013).
Stoll, S. & Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
MATLAB (The Mathworks Inc., 2015).
Krieger, E. & Vriend, G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 36, 996–1007 (2015).
Tian, C. et al. ff19SB: amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theory Comput. 16, 528–552 (2020).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general AMBER force field. J. Comput. Chem. 25, 1157–1174 (2004).
Acknowledgements
We thank the Czech Science Foundation (grant no. GA22-09853S) and the Czech Ministry of Education (INBIO grant nos. CZ.02.1.01/0.0/0.0/16_026/0008451, RECETOX RI LM2018121 and e-INFRA LM2018140). This project has received funding from the European Union’s Horizon 2020 research and innovation programme (grant nos. TEAMING 857560 and Sinfonia 814418) and the Marie Sklodowska-Curie Action (grant no. 792772). M.M. acknowledges financial support from GAMU of the Masaryk University (grant no. MUNI/H/1561/2018) and M.T. is a Brno PhD Talent Scholarship holder funded by the Brno City Municipality. CIISB research infrastructure project (grant no. LM2018127) is acknowledged for financial support of the measurements at Biomolecular Interactions and Crystallization Core Facility. P.C. acknowledges research support from VISTEC, TSRI and the NSRF via the Programme Management Unit for Human Resources & Institutional Development, Research and Innovation grant no. B05F640089 and Kasikorn Bank. P.N. acknowledges European Research Council grant (no. 714850) under the European Union´s Horizon 2020 research and innovation programme. G.G. acknowledges a PhD fellowship from the University of Paris Descartes, Sorbonne Paris City. The design and synthesis of azaCTZ also benefited from the Valoexpress funding calls of the Institut Pasteur. The crystallographic experiments were performed using the PXIII beamline at the Swiss Light Source (SLS) in Villigen (Switzerland). We are grateful to the members of the SLS synchrotron for the use of their beamline and help during data collection.
Author information
Authors and Affiliations
Contributions
R.B., G.G. and Y.L.J. designed, synthesized and characterized azaCTZ. A.S., M.S. and M.M. constructed and cloned DNA mutants and produced all recombinant proteins. D.P., A.S. and Z.P. performed luciferase and steady-state kinetics assays. A.S. and M.M. performed cocrystallization experiments, collected X-ray data and solved protein–ligand structures. M.T. and Z.P. carried out high-performance liquid chromatography experiments. V.T.S., A.S., M.T., M.M. and P.N. conducted EPR experiments. M.T. and Z.P. performed oxygen-free assays and spectral experiments. G.P.P., J.D. and D.B. performed and analysed molecular dynamics simulations. P.C. contributed to experimental design and data interpretation. M.M., Z.P. and J.D. designed the study and contributed to data interpretation. All authors contributed to the writing of the manuscript and preparation of tables and figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Naohiro Kato, Per-Olof Syren, Roberto Steiner, Anderson Garbuglio Oliveira and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, Notes 1–3, Tables 1–8 and nuclear magnetic resonance spectra.
Supplementary Data
Source data for all relevant Supplementary Figures.
Source data
Source Data Fig. 1
Raw data for Fig.1c,d.
Source Data Fig. 4
Raw data and source data used for graph plotting.
Source Data Fig. 5
Raw data and source data used for graph plotting.
Source Data Fig. 6
Raw data and source data used for graph plotting.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schenkmayerova, A., Toul, M., Pluskal, D. et al. Catalytic mechanism for Renilla-type luciferases. Nat Catal 6, 23–38 (2023). https://doi.org/10.1038/s41929-022-00895-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00895-z
This article is cited by
-
Illuminating the mechanism and allosteric behavior of NanoLuc luciferase
Nature Communications (2023)
-
Loop dynamics and the evolution of enzyme activity
Nature Reviews Chemistry (2023)
-
The crucial role of Y109 and R162 as catalytic residues of nanoKAZ: insights from molecular docking, molecular dynamics simulation, and quantum chemical investigations
Journal of Molecular Modeling (2023)