Abstract
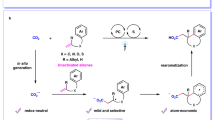
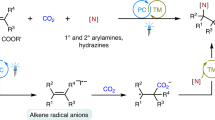
High value utilization of carbon dioxide (CO2) has attracted worldwide attention for decades. Catalytic carboxylation of alkenes with CO2 to synthesize valuable carboxylic acids and diacids is highly important. Although visible-light photocatalytic single-electron transfer reduction of CO2 could provide an alternative choice for diverse chemo- and regio-selectivities, it has rarely been investigated in carboxylation. Moreover, visible-light photocatalytic carboxylation of unactivated alkenes with CO2•− has never been reported. Here we report visible-light photocatalytic di- and hydro-carboxylation of unactivated alkenes with CO2. In contrast to previous reports limited to activated alkenes, diverse unactivated aliphatic alkenes undergo selective carboxylations to give carboxylic acids, dicarboxylic acids and unnatural α-amino acid derivatives in moderate to good yields. Mechanistic studies suggest that CO2 might be reduced to CO2•− via consecutive photo-induced electron transfer, and this species would attack unactivated alkenes followed by subsequent hydrogen atom transfer and other relevant processes to afford the corresponding products.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Details about materials and methods, experimental procedures, mechanistic studies, characterization data and NMR spectra are available in the Supplementary Information. Additional data are available from the corresponding author upon reasonable request.
References
Ciamician, G. The photochemistry of the future. Science 36, 385–394 (1912).
Stephenson, C., Yoon, T. & MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry (Wiley-VCH, 2018).
Goddard, J.-P., Ollivier, C. & Fensterbank, L. Photoredox catalysis for the generation of carbon centered radicals. Acc. Chem. Res. 49, 1924–1936 (2016).
Hopkinson, M. N., Tlahuext-Aca, A. & Glorius, F. Merging visible light photoredox and gold catalysis. Acc. Chem. Res. 49, 2261–2272 (2016).
Ravelli, D., Protti, S. & Fagnoni, M. Carbon–carbon bond forming reactions via photogenerated intermediates. Chem. Rev. 116, 9850–9913 (2016).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Gentry, E. C. & Knowles, R. R. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 49, 1546 (2016).
Liu, Q. & Wu, L.-Z. Recent advances in visible-light-driven organic reactions. Natl Sci. Rev. 4, 359–380 (2017).
Marzo, L., Pagire, S. K., Reiser, O. & König, B. Visible-light photocatalysis: does it make a difference in organic synthesis? Angew. Chem. Int. Ed. 57, 10034–10072 (2018).
Chen, Y., Lu, L.-Q., Yu, D.-G., Zhu, C.-J. & Xiao, W.-J. Visible light-driven organic photochemical synthesis in China. Sci. China Chem. 62, 24–57 (2019).
Liu, Q., Wu, L., Jackstell, R. & Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 6, 5933 (2015).
Tortajada, A., Börjesson, M. & Martin, R. Nickel-catalyzed reductive carboxylation and amidation reactions. Acc. Chem. Res. 54, 3941–3952 (2021).
Cao, Y., He, X., Wang, N., Li, H.-R. & He, L.-N. Photochemical and electrochemical carbon dioxide utilization with organic compounds. Chin. J. Chem. 36, 644–659 (2018).
Yeung, C. S. Photoredox catalysis as a strategy for CO2 incorporation: direct access to carboxylic acids from a renewable feedstock. Angew. Chem. Int. Ed. 58, 5492–5502 (2019).
Cai, B., Cheo, H. W., Liu, T. & Wu, J. Light-promoted organic transformations utilizing carbon-based gas molecules as feedstocks. Angew. Chem. Int. Ed. 60, 2–33 (2021).
Ye, J.-H., Ju, T., Huang, H., Liao, L.-L. & Yu, D.-G. Radical carboxylative cyclizations and carboxylations with CO2. Acc. Chem. Res. 54, 2518–2531 (2021).
Klankermayer, J., Wesselbaum, S., Beydoun, K. & Leitner, W. Selective catalytic synthesis using the combination of carbon dioxide and hydrogen: catalytic chess at the interface of energy and chemistry. Angew. Chem. Int. Ed. 55, 7296–7343 (2016).
He, M., Sun, Y. & Han, B. Green carbon science: scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. 52, 9620–9633 (2013).
Grignard, B., Gennen, S., Jérôme, C., Kleij, A. W. & Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 48, 4466–4514 (2019).
Matthessen, R., Fransaer, J., Binnemans, K. & Vos, D. E. D. Electrocarboxylation: towards sustainable and efficient synthesis of valuable carboxylic acids. Beilstein J. Org. Chem. 10, 2484–2500 (2014).
Seo, H., Katcher, M. H. & Jamison, T. F. Photoredox activation of carbon dioxide for amino acid synthesis in continuous flow. Nat. Chem. 9, 453–456 (2016).
Alkayal, A. et al. Harnessing applied potential: selective β-hydrocarboxylation of substituted olefins. J. Am. Chem. Soc. 142, 1780–1785 (2020).
Murata, K., Numasawa, N., Shimomaki, K., Takaya, J. & Iwasawa, N. Construction of a visible light-driven hydrocarboxylation cycle of alkenes by the combined use of Rh(I) and photoredox catalysts. Chem. Commun. 53, 3098–3101 (2017).
Yatham, V. R., Shen, Y. & Martin, R. Catalytic intermolecular dicarbofunctionalization of styrenes with CO2 and radical precursors. Angew. Chem. Int. Ed. 56, 10915–10919 (2017).
Ye, J.-H. et al. Visible-light-driven iron-promoted thiocarboxylation of styrenes and acrylates with CO2. Angew. Chem. Int. Ed. 56, 15416–15420 (2017).
Meng, Q.-Y., Wang, S., Huff, G. S. & König, B. Ligand-controlled regioselective hydrocarboxylation of styrenes with CO2 by combining visible light and nickel catalysis. J. Am. Chem. Soc. 140, 3198–3201 (2018).
Hou, J. et al. Visible-light-mediated metal-free difunctionalization of alkenes with CO2 and silanes or C(sp3)−H alkanes. Angew. Chem. Int. Ed. 57, 17220–17224 (2018).
Wang, H., Gao, Y., Zhou, C. & Li, G. Visible-light-driven reductive carboarylation of styrenes with CO2 and aryl halides. J. Am. Chem. Soc. 142, 8122–8129 (2020).
Ju, T. et al. Dicarboxylation of alkenes, allenes, and (hetero)arenes with CO2 via visible-light photoredox catalysis. Nat. Catal. 4, 304–311 (2021).
Juhl, M. et al. Copper-catalyzed carboxylation of hydroborated disubstituted alkenes and terminal alkynes with cesium fluoride. ACS Catal. 7, 1392–1396 (2017).
Gaydou, M., Moragas, T., Juliá-Hernández, F. & Martin, R. Site-selective catalytic carboxylation of unsaturated hydrocarbons with CO2 and water. J. Am. Chem. Soc. 139, 12161–12164 (2017).
Song, L. et al. Visible-light photoredox-catalyzed remote difunctionalizing carboxylation of unactivated alkenes with CO2. Angew. Chem. Int. Ed. 59, 21121–21128 (2020).
Cybularczyk-Cecotka, M., Szczepanik, J. & Giedyk, M. Photocatalytic strategies for the activation of organic chlorides. Nat. Catal. 3, 872–886 (2020).
Concepcion, J. J., House, R. L., Papanikolas, J. M. & Meyer, T. J. Chemical approaches to artificial photosynthesis. Proc. Natl Acad. Sci. USA 109, 15560–15564 (2012).
La Porte, N. T. et al. Photoexcited radical anion super-reductants for solar fuels catalysis. Coord. Chem. Rev. 361, 98–119 (2018).
Glaser, F., Kerzig, C. & Wenger, O. S. Multi-photon excitation in photoredox catalysis: concepts, applications, methods. Angew. Chem. Int. Ed. 59, 10266–10284 (2020).
Schreier, M. R. et al. Water-soluble tris(cyclometalated) iridium(III) complexes for aqueous electron and energy transfer photochemistry. Acc. Chem. Res. 55, 1290–1300 (2022).
Liao, L.-L., Song, L., Yan, S.-S., Ye, J.-H. & Yu, D.-G. Highly reductive photocatalytic systems in organic synthesis. Trends Chem. 4, 512–527 (2022).
Neumeier, M. et al. Dichromatic photocatalytic substitutions of aryl halides with a small organic dye. Chem. Eur. J. 24, 105–108 (2018).
Ghosh, I., Ghosh, T., Bardagi, J. I. & König, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science 346, 725–728 (2014).
Kerzig, C., Guo, X. & Wenger, O. S. Unexpected hydrated electron source for preparative visible-light driven photoredox catalysis. J. Am. Chem. Soc. 141, 2122–2127 (2019).
Giedyk, M. et al. Photocatalytic activation of alkyl chlorides by assembly-promoted single electron transfer in microheterogeneous solutions. Nat. Catal. 3, 40–47 (2020).
MacKenzie, I. A. et al. Discovery and characterization of an acridine radical photoreductant. Nature 580, 76–80 (2020).
Xu, J. et al. Unveiling extreme photoreduction potentials of donor–acceptor cyanoarenes to access aryl radicals from aryl chlorides. J. Am. Chem. Soc. 143, 13266–13273 (2021).
Cole, J. P. et al. Organocatalyzed Birch reduction driven by visible light. J. Am. Chem. Soc. 142, 13573–13581 (2020).
Rosso, J. A., Bertolotti, S. G., Braun, A. M., Mártire, D. O. & Gonzalez, M. C. Reactions of carbon dioxide radical anion with substituted benzenes. J. Phys. Org. Chem. 14, 300–309 (2001).
Hendy, C. M., Smith, G. C., Xu, Z., Lian, T. & Jui, N. T. Radical chain reduction via carbon dioxide radical anion (CO2•−). J. Am. Chem. Soc. 143, 8987–8992 (2021).
Yan, S.-S. et al. Visible-light photoredox-catalyzed selective carboxylation of C(sp3)−F bonds with CO2. Chem 7, 3099–3113 (2021).
Chmiel, A. F., Williams, O. P., Chernowsky, C. P., Yeung, C. S. & Wickens, Z. K. Non-innocent radical ion intermediates in photoredox catalysis: parallel reduction modes enable coupling of diverse aryl chlorides. J. Am. Chem. Soc. 143, 10882–10889 (2021).
Morgenstern, D. A., Wittrig, R. E., Fanwick, P. E. & Kubiak, C. P. Photoreduction of carbon dioxide to its radical anion by [Ni3(μ3-I)2(dppm)3]: formation of two carbon–carbon bonds via addition of CO2•− to cyclohexene. J. Am. Chem. Soc. 115, 6470–6471 (1993).
Alektiar, S. N. & Wickens, Z. K. Photoinduced hydrocarboxylation via thiol-catalyzed delivery of formate across activated alkenes. J. Am. Chem. Soc. 143, 13022–13028 (2021).
Huang, H. et al. Visible light-driven anti-Markovnikov hydrocarboxylation of acrylates and styrenes with CO2. CCS Chem. 2, 1746–1756 (2020).
Stateman, L. M., Nakafuku, K. M. & Nagib, D. A. Remote C–H functionalization via selective hydrogen atom transfer. Synthesis 50, 1569–1586 (2018).
Li, W., Xu, W., Xie, J., Yu, S. & Zhu, C. Distal radical migration strategy: an emerging synthetic means. Chem. Soc. Rev. 47, 654–667 (2018).
Acknowledgements
We thank O. S. Wenger, F. Glaser and B. Pfund from the University of Basel and J. J. Chruma from the University of Virginia for valuable suggestions. Financial support is provided by the National Natural Science Foundation of China (22225106, 21822108), Sichuan Science and Technology Program (20CXTD0112), Central Government Funds of Guiding Local Scientific and Technological Development for Sichuan Province (2021ZYD0063), Fundamental Research Funds from Sichuan University (2020SCUNL102) and the Fundamental Research Funds for the Central Universities. We also thank X. Wang, H. Chen and Y. Luo from the Analysis and Testing Center of Sichuan University as well as J. Li, Q.-F. Zhang, D. Deng, L. Wei and Y. Long from the College of Chemistry at Sichuan University for analytic testing and valuable help.
Author information
Authors and Affiliations
Contributions
D.-G.Y. conceived, designed and supervised the study. L.S., W.W. and D.-G.Y. wrote the paper. L.S., W.W., J.-P.Y., Y.-X.J., M.-K.W., H.-P.Z., S.-S.Y. and L.-L.L. performed the experiments and mechanistic studies. L.S. and W.W. contributed equally to this work. All authors contributed to the analysis and interpretation of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing financial interest(s): two Chinese Patents on this work have been applied for with the numbers 202111106015.2 (D.-G.Y., L.S., M.-K.W. and Y.-X.J.) and 202210530049.2 (D.-G.Y., W.W., J.-P.Y., L.S., H.-P.Z., S.-S.Y. and L.-L.L.).
Peer review
Peer review information
Nature Catalysis thanks Zachary K. Wickens, Liangnian He and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, references, Figs. 1–40 and Tables 1–10.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, L., Wang, W., Yue, JP. et al. Visible-light photocatalytic di- and hydro-carboxylation of unactivated alkenes with CO2. Nat Catal 5, 832–838 (2022). https://doi.org/10.1038/s41929-022-00841-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00841-z
This article is cited by
-
Electrophotocatalytic hydrogenation of imines and reductive functionalization of aryl halides
Nature Communications (2024)
-
Straightforward synthesis of functionalized γ-Lactams using impure CO2 stream as the carbon source
Nature Communications (2023)
-
Photocatalytic CO2 reduction
Nature Reviews Methods Primers (2023)
-
Arylcarboxylation of unactivated alkenes with CO2 via visible-light photoredox catalysis
Nature Communications (2023)