Abstract
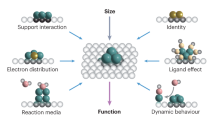
A large family of heterogeneous catalytic reactions require active sites with more than one metal atom, that is, an ensemble of metal atoms. The ensemble requirement, which refers to the minimum number of metal atoms that are needed to catalyse a reaction with optimal efficiency, is a useful metric to evaluate the effectiveness of catalysts for reactions with different site requirements. In this Review, we revisit the traditional ensemble effect and lay out the principles for its incorporation within efficient metal catalysts. Single-atom catalysts can also be described through the ensemble effect theory, as the coordination groups of single-atom catalysts constitute an ensemble that is vital for their reactivity. The understanding of the ensemble requirement for metal catalysts provides insights into catalyst design with both optimized activity and atomic efficiency, and contributes to the development of sustainable heterogeneous catalytic transformations.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
14 October 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41929-022-00869-1
References
Taylor, H. S. A theory of catalytic surface. Proc. R. Soc. Lond. A 108, 105–111 (1925).
Vogt, C. & Weckhuysen, B. M. The concept of active site in heterogeneous catalysis. Nat. Rev. Chem 6, 89–111 (2022). The authors introduce the historical understanding of active sites in heterogeneous catalysis, analyse the functions of different types of active sites and give prospects for the design of active sites.
Hoffman, A. S. et al. Beating heterogeneity of single-site catalysts: MgO-supported iridium complexes. ACS Catal. 8, 3489–3498 (2018).
Hoffman, A. S., Fang, C. & Gates, B. C. Homogeneity of surface sites in supported single-site metal catalysts: assessment with band widths of metal carbonyl infrared spectra. J. Phys. Chem. Lett. 7, 3854–3860 (2016).
Andersson, M. P. et al. Structure sensitivity of the methanation reaction: H2-induced CO dissociation on nickel surfaces. J. Catal. 255, 6–19 (2008).
Lauritsen, J. V., Vang, R. T. & Besenbacher, F. From atom-resolved scanning tunneling microscopy (STM) studies to the design of new catalysts. Catal. Today 111, 34–43 (2006).
Schwartz, T. J. et al. Engineering catalyst microenvironments for metal-catalyzed hydrogenation of biologically derived platform chemicals. Angew. Chem. Int. Ed. 53, 12718–12722 (2014).
Studt, F. et al. Discovery of a Ni–Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 6, 320–324 (2014).
Li, W. et al. Chemical insights into the design and development of face-centered cubic ruthenium catalysts for Fischer–Tropsch synthesis. J. Am. Chem. Soc. 139, 2267–2276 (2017).
Lykhach, Y. et al. Counting electrons on supported nanoparticles. Nat. Mater. 15, 284–288 (2016).
Jiang, L. et al. Facet engineering accelerates spillover hydrogenation on highly diluted metal nanocatalysts. Nat. Nanotechnol. 15, 848–853 (2020).
Bara, C. et al. Aqueous-phase preparation of model HDS catalysts on planar alumina substrates: support effect on Mo adsorption and sulfidation. J. Am. Chem. Soc. 137, 15915–15928 (2015).
Ye, T. et al. Vacancy-enabled N2 activation for ammonia synthesis on an Ni-loaded catalyst. Nature 583, 391–395 (2020).
Qi, J. et al. Selective methanol carbonylation to acetic acid on heterogeneous atomically dispersed ReO4/SiO2 catalysts. J. Am. Chem. Soc. 142, 14178–14189 (2020).
Somorjai, G. A. & Carrazza, J. Structure sensitivity of catalytic reactions. Ind. Eng. Chem. Fund. 25, 63–69 (1986).
Wachs, I. E. Number of surface sites and turnover frequencies for oxide catalysts. J. Catal. 405, 462–472 (2022).
Dong, C. et al. Supported metal clusters: fabrication and application in heterogeneous catalysis. ACS Catal. 10, 11011–11045 (2020).
Honkala, K. et al. Ammonia synthesis from first-principles calculations. Science 307, 555–558 (2005). The authors perform DFT calculations to predict the reaction rates of N2 reduction on Ru particle catalysts, in which Ru particles larger than 2 nm are able to provide step sites for N2 dissociation.
Peterson, A. A. et al. Finite-size effects in O and CO adsorption for the late transition metals. Top. Catal. 55, 1276–1282 (2012).
Sachtler, W. M. H. & van Santen, R. A. Surface composition and selectivity of alloy catalysts. Adv. Catal. 26, 69–119 (1977). The authors summarize the ensemble effect for different chemical reactions on the surface of a metal alloy, one of the earliest research papers on the ensemble effect.
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Dong, C. et al. Fully exposed palladium cluster catalysts enable hydrogen production from nitrogen heterocycles. Nat. Catal. 5, 485–493 (2022).
Peng, M. et al. Fully exposed cluster catalyst (FECC): toward rich surface sites and full atom utilization efficiency. ACS Cent. Sci. 7, 262–273 (2021). Fully exposed metal clusters not only adapt to the ensemble requirement for many reactions, but also ensure a high metal utilization and have more types of surface sites.
Van Santen, R. A. Complementary structure sensitive and insensitive catalytic relationships. Acc. Chem. Res. 42, 57–66 (2009).
Den Breejen, J. P. et al. On the origin of the cobalt particle size effects in Fischer–Tropsch catalysis. J. Am. Chem. Soc. 131, 7197–7203 (2009).
Jacobsen, C. J. H. et al. Structure sensitivity of supported ruthenium catalysts for ammonia synthesis. J. Mol. Catal. A: Chem. 163, 19–26 (2000).
Newton, M. A., Knorpp, A. J., Sushkevich, V. L., Palagin, D. & van Bokhoven, J. A. Active sites and mechanisms in the direct conversion of methane to methanol using Cu in zeolitic hosts: a critical examination. Chem. Soc. Rev. 49, 1449–1486 (2020).
Woertink, J. S. A. [Cu2O]2+ core in Cu-ZSM-5, the active site in the oxidation of methane to methanol. Proc. Natl Acad. Sci. USA 106, 18908–18913 (2009).
Zhang, L., Zhou, M., Wang, A. & Zhang, T. Selective hydrogenation over supported metal catalysts: from nanoparticles to single atoms. Chem. Rev. 120, 683–733 (2020).
Guan, E. et al. MgO-supported iridium metal pair-site catalysts are more active and resistant to CO poisoning than analogous single-site catalysts for ethylene hydrogenation and hydrogen–deuterium exchange. ACS Catal. 9, 9545–9553 (2019).
Huang, F. et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene. J. Am. Chem. Soc. 140, 13142–13146 (2018).
Masson, A. et al. Intrinsic size effect of platinum particles supported on plasma-grown amorphous alumina in the hydrogenation of ethylene. Surf. Sci. 173, 479–497 (1986).
Zhang, X. et al. Structure sensitivity of n-butane hydrogenolysis on supported Ir catalysts. J. Catal. 394, 376–386 (2021).
Zhang, S. et al. Insights into the mechanism of n-hexane reforming over a single-site platinum catalyst. J. Am. Chem. Soc. 142, 16533–16537 (2020).
Jeong, H. et al. Fully dispersed Rh ensemble catalyst to enhance low-temperature activity. J. Am. Chem. Soc. 140, 9558–9565 (2018).
Jeong, H. et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 3, 368–375 (2020).
Giannakakis, G. et al. NiAu single atom alloys for the non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. Top. Catal. 61, 475–486 (2018).
Ouyang, M. et al. Directing reaction pathways via in situ control of active site geometries in PdAu single-atom alloy catalysts. Nat. Commun. 12, 1549 (2021).
Mavrikakis, M. & Barteau, M. A. Oxygenate reaction pathways on transition metal surfaces. J. Mol. Catal. A: Chem. 131, 135–147 (1998).
Sinfelt, J. H. Bimetallic Catalysts: Discoveries, Concepts, and Applications (Wiley & Sons, Inc., 1983).
Lei, G. & Sachtler, W. M. H. H/D exchange of cyclopentane of Pt/mordenites: probing the monoatomic Pt sites. J. Catal. 140, 601–611 (1993). Taking advantage of the fact that a single-atom Pt catalyst cannot complete multiple H–D exchanges in cyclopentane, Pt ensembles and single Pt sites are clearly distinguished by the different results in the H–D exchange experiments of cyclopentane.
Zholobenko, V., Lei, G., Carvill, B. T., Lerner, B. A. & Sachtler, W. M. H. Identification of isolated Pt atoms in H-mordenite. J. Chem. Soc. Faraday Trans. 90, 233–238 (1994).
Campbell, C. T. et al. Probing ensemble effects in surface reactions. 1. Site-size requirements for the dehydrogenation of cyclic hydrocarbons on Pt(111) revealed by bismuth site blocking. J. Phys. Chem. 93, 806–814 (1989).
Desai, P. H. & Richardson, J. T. Crystallite size effects in nickel catalysts: cyclohexane dehydrogenation and hydrogenolysis. J. Catal. 98, 392–400 (1986).
Shi, H., Li, X., Haller, G. L., Gutiérrez, O. Y. & Lercher, J. A. Active sites and reactive intermediates in the hydrogenolytic cleavage of C–C bonds in cyclohexane over supported iridium. J. Catal. 295, 133–145 (2012).
Deng, Y. et al. Few-atom Pt ensembles enable efficient catalytic cyclohexane dehydrogenation for hydrogen production. J. Am. Chem. Soc. 144, 3535–3542 (2022).
Sachtler, W. M. H. in Handbook of Heterogeneous Catalysis Ch. 5 (eds Ertl, G. et al.) 1585–1593 (Wiley-VCH, 2008).
Che, M. & Bennett, C. O. The influence of particle size on catalytic properties of supported metals. Adv. Catal. 36, 55–172 (1989).
Liu, P. & Nørskov, J. K. Ligand and ensemble effects in adsorption on alloy surfaces. Phys. Chem. Chem. Phys. 3, 3814–3818 (2001).
Nilsson, A., Pettersson, L. G. M. & Nørskov J. K. Chemical Bonding at Surfaces and Interfaces (Elsevier, 2007).
Liu, L. et al. Determination of the evolution of heterogeneous single metal atoms and nanoclusters under reaction conditions: which are the working catalytic sites? ACS Catal. 9, 10626–10639 (2019).
Ding, K. et al. Identification of active sites in CO oxidation and water–gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Heiz, U., Sanchez, A., Abbet, S. & Schnerder, W. D. Catalytic oxidation of carbon monoxide on monodispersed platinum clusters: each atom counts. J. Am. Chem. Soc. 121, 3214–3217 (1999).
Bamwenda, G. R., Tsubota, S., Nakamura, T. & Haruta, M. The influence of the preparation methods on the catalytic activity of platinum and gold supported on TiO2 for CO oxidation. Catal. Lett. 44, 83–87 (1997).
Zhou, X. et al. Stable Pt single atoms and nanoclusters on ultrathin CuO film and their performances in CO oxidation. J. Phys. Chem. C 120, 1709–1715 (2016).
Maurer, F. et al. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nat. Catal. 3, 824–833 (2020). In situ EXAFS is used to reveal the dynamics of single-atom Pt/CeO2 catalyst during CO oxidation, in which the catalytically active species are verified to be Pt ensembles rather than single-atom Pt.
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Muravev, V. et al. Interface dynamics of Pd-CeO2 single-atom catalysts during CO oxidation. Nat. Catal. 4, 469–478 (2021).
DeRita, L. et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 139, 14150–14165 (2017).
Lin, B., Wei, K., Lin, J. & Ni, J. Effect of treatment conditions on ruthenium particle size and ammonia synthesis activity of ruthenium catalyst. Catal. Commun. 39, 14–19 (2013).
Li, Z. et al. Ammonia synthesis on graphitic-nanofilament supported Ru catalysts. J. Mol. Catal. A: Chem. 211, 103–109 (2004).
Ishikawa, A., Doi, T. & Nakai, H. Catalytic performance of Ru, Os, and Rh nanoparticles for ammonia synthesis: a density functional theory analysis. J. Catal. 357, 213–222 (2018).
Van Hardeveld, R. & Hartog, F. The statistics of surface atoms and surface sites on metal crystals. Surf. Sci. 15, 189–230 (1969).
Zhang, B., Su, H., Liu, J. & Li, W. Interplay between site activity and density of BCC iron for ammonia synthesis based on first-principles theory. ChemCatChem 11, 1928–1934 (2019).
Guo, X. et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 344, 616–619 (2014).
Marcinkowski, M. D. et al. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C–H activation. Nat. Chem. 10, 325–332 (2018).
Vilé, G. et al. A stable single-site palladium catalyst for hydrogenations. Angew. Chem. Int. Ed. 54, 11265–11269 (2015).
Lin, J. et al. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 135, 15314–15317 (2013).
Nie, L. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Lin, L. et al. Atomically dispersed Ni/α-MoC catalyst for hydrogen production from methanol/water. J. Am. Chem. Soc. 143, 309–317 (2021).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017). The synergy between α-MoC support and atomic Pt sites makes it possible to dissociate water on α-MoC and activate methanol on Pt, to achieve an outstanding activity of methanol-water reforming at low temperature.
Chen, A. et al. Structure of the catalytically active copper–ceria interfacial perimeter. Nat. Catal. 2, 334–341 (2019).
Cao, L. et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2. Nature 565, 631–635 (2019).
Kahlich, M. J., Gasteiger, H. A. & Behm, R. J. Kinetics of the selective CO oxidation in H2-rich gas on Pt/Al2O3. J. Catal. 171, 93–105 (1997).
Allian, A. D. et al. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 133, 4498–4517 (2011).
Zhai, Y. et al. Alkali-stabilized Pt-OHx species catalyze low-temperature water-gas shift reactions. Science 329, 1633–1636 (2010). Na+ additive in Pt/SiO2 catalyst assists the dissociation of water and provides a large amount of OH group, which helps to activate CO on Pt centres and leads to a much higher water-gas shift reaction activity at low temperatures as compared with that of the Pt/SiO2 catalyst with no alkali metal additives.
Yang, M. et al. Catalytically active Au-O(OH)x species stabilized by alkali ions on zeolites and mesoporous oxides. Science 346, 1498–1501 (2014).
Qin, R. et al. Alkali ions secure hydrides for catalytic hydrogenation. Nat. Catal. 3, 703–709 (2020).
Wang, J. et al. N-coordinated dual-metal single-site catalyst for low-temperature CO oxidation. ACS Catal. 10, 2754–2761 (2020).
Fu, J. et al. Synergistic effects for enhanced catalysis in a dual single-atom catalyst. ACS Catal. 11, 1952–1961 (2021).
Aich, P. et al. Single-atom alloy Pd–Ag catalyst for selective hydrogenation of acrolein. J. Phys. Chem. C 119, 18140–18148 (2015).
Liu, P. & Zheng, N. Coordination chemistry of atomically dispersed catalysts. Natl. Sci. Rev. 5, 636–638 (2018).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Vercammen, J. et al. Shape-selective C–H activation of aromatics to biarylic compounds using molecular palladium in zeolites. Nat. Catal. 3, 1002–1009 (2020).
Tian, S. et al. Carbon nitride supported Fe2 cluster catalysts with superior performance for alkene epoxidation. Nat. Commun. 9, 2353 (2018).
Pan, Y. et al. Regulating the coordination structure of single-atom Fe-NxCy catalytic sites for benzene oxidation. Nat. Commun. 10, 4290 (2019).
Gan, T. et al. Facile synthesis of kilogram-scale Co-alloyed Pt single-atom catalysts via ball milling for hydrodeoxygenation of 5-hydroxymethylfurfural. ACS Sustainable Chem. Eng. 8, 8692–8699 (2020).
Shimizu, K., Miyamoto, Y. & Satsuma, A. Size- and support-dependent silver cluster catalysis for chemoselective hydrogenation of nitroaromatics. J. Catal. 270, 86–94 (2010).
Boronat, M. et al. A molecular mechanism for the chemoselective hydrogenation of substituted nitroaromatics with nanoparticles of gold on TiO2 catalysts: a cooperative effect between gold and the support. J. Am. Chem. Soc. 129, 16230–16237 (2007).
Zečević, J., Vanbutsele, G., de Jong, K. P. & Martens, J. A. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature 528, 245–248 (2015).
Resasco, J. & Christopher, P. Atomically dispersed Pt-group catalysts: reactivity, uniformity, structural evolution, and paths to increased functionality. J. Phys. Chem. Lett. 11, 10114–10123 (2020).
Zhang, X. et al. Reversible loss of core–shell structure for Ni–Au bimetallic nanoparticles during CO2 hydrogenation. Nat. Catal. 3, 411–417 (2020).
Feng, S. et al. In situ formation of mononuclear complexes by reaction-induced atomic dispersion of supported noble metal nanoparticles. Nat. Commun. 10, 5281 (2019).
Zhai, H. & Alexandrova, A. N. Fluxionality of catalytic clusters: when it matters and how to address it. ACS Catal. 7, 1905–1911 (2017).
Sun, Q. et al. Subnanometer bimetallic platinum–zinc clusters in zeolites for propane dehydrogenation. Angew. Chem. Int. Ed. 59, 19450–19459 (2020).
Zhang, W. et al. Size dependence of Pt catalysts for propane dehydrogenation: from atomically dispersed to nanoparticles. ACS Catal. 10, 12932–12942 (2020).
Ledesma, C., Yang, J., Chen, D. & Holmen, A. Recent approaches in mechanistic and kinetic studies of catalytic reactions using SSITKA technique. ACS Catal. 4, 4527–4547 (2014).
Harding, D. J. et al. Ion and velocity map imaging for surface dynamics and kinetics. J. Chem. Phys. 147, 013939 (2017).
Borodin, D. et al. Measuring transient reaction rates from nonstationary catalysts. ACS Catal. 10, 14056–14066 (2020).
Zaera, F. Use of molecular beams for kinetic measurements of chemical reactions on solid surfaces. Surf. Sci. Rep. 72, 59–104 (2017).
Acknowledgements
This work received financial support from the National Key R&D Program of China (2021YFA1501100), the Natural Science Foundation of China (21725301, 21932002, 22005007 and 21821004), and the China Petrochemical Corporation (Sinopec Group) (project no. 122085). D.M. acknowledges support from the Tencent Foundation through the XPLORER PRIZE. Y.G. acknowledges support from the China Postdoctoral Science Foundation (no. 2020M680195) and Beijing Molecular Sciences Junior Fellow Program.
Author information
Authors and Affiliations
Contributions
Y.G., M.W. and D.M. wrote the first draft of the paper, and Q.Z. and D.X. revised the manuscript. Specifically, D.M. and Q.Z. drew the outline of the manuscript, M.W. and Y.G. wrote the PME effect part and Y.G. wrote the HAE effect part. The rest of the article was written by all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Abhaya Datye and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Y., Wang, M., Zhu, Q. et al. Ensemble effect for single-atom, small cluster and nanoparticle catalysts. Nat Catal 5, 766–776 (2022). https://doi.org/10.1038/s41929-022-00839-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00839-7
This article is cited by
-
Emerging Atomically Precise Metal Nanoclusters and Ultrasmall Nanoparticles for Efficient Electrochemical Energy Catalysis: Synthesis Strategies and Surface/Interface Engineering
Electrochemical Energy Reviews (2024)
-
Building Feedback-Regulation System Through Atomic Design for Highly Active SO2 Sensing
Nano-Micro Letters (2024)
-
Advances in heterogeneous single-cluster catalysis
Nature Reviews Chemistry (2023)
-
Evidence of bifunctionality of carbons and metal atoms in catalyzed acetylene hydrochlorination
Nature Communications (2023)
-
Language models and protocol standardization guidelines for accelerating synthesis planning in heterogeneous catalysis
Nature Communications (2023)