Abstract
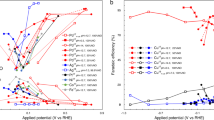
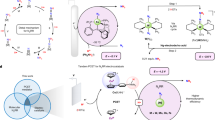
The selective transformation of nitrogen compounds is a foundation of the modern chemical industry. Existing thermochemical processes largely rely on fossil fuels and innovating electrocatalytic processes that could use renewable energy remains challenging. Here we report the electrochemical regulation of a nitrite reduction network using a molybdenum sulfide catalyst by modulating the thermodynamic driving force of proton and electron transfer. The strategy behind this approach is based on the theory of sequential proton–electron transfer, in which the driving force of proton and electron transfer can be optimized independently. This makes it possible to target the desired reactions with selectivities of up to 80% for NO, 61% for N2O, 36% for N2 and 100% for NH4+, comparable to the highest values reported to date using a specific catalyst optimized for a single target product. Consistency with numerical simulation highlights that sequential proton–electron transfer can be used to rationally regulate the electrochemical nitrogen network.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. The Python program for numerical simulations can be found at GitHub (https://github.com/HideshiOoka/SI_for_Publications).
References
Chen, J. G. et al. Beyond fossil fuel-driven nitrogen transformations. Science 360, eaar6611 (2018).
Lehnert, N., Musselman, B. W. & Seefeldt, L. C. Grand challenges in the nitrogen cycle. Chem. Soc. Rev. 50, 3640–3646 (2021).
Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. Nitrogen cycle electrocatalysis. Chem. Rev. 109, 2209–2244 (2009).
Kuypers, M. M. M., Marchant, H. K. & Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 16, 263–276 (2018).
Lehnert, N., Dong, H. T., Harland, J. B., Hunt, A. P. & White, C. J. Reversing nitrogen fixation. Nat. Rev. Chem. 2, 278–289 (2018).
Erisman, J. W., Sutton, M. A., Galloway, J., Klimont, Z. & Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639 (2008).
Wang, Y., Wang, C., Li, M., Yu, Y. & Zhang, B. Nitrate electroreduction: mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 50, 6720–6733 (2021).
Duca, M. & Koper, M. T. M. Powering denitrification: the perspectives of electrocatalytic nitrate reduction. Energy Environ. Sci. 5, 9726–9742 (2012).
van Langevelde, P. H., Katsounaros, I. & Koper, M. T. M. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule 5, 290–294 (2021).
MacFarlane, D. R. et al. A roadmap to the ammonia economy. Joule 4, 1186–1205 (2020).
Braley, S. E., Xie, J., Losovyj, Y. & Smith, J. M. Graphite conjugation of a macrocyclic cobalt complex enhances nitrite electroreduction to ammonia. J. Am. Chem. Soc. 143, 7203–7208 (2021).
Uyeda, C. & Peters, J. C. Selective nitrite reduction at heterobimetallic CoMg complexes. J. Am. Chem. Soc. 135, 12023–12031 (2013).
Guo, Y., Stroka, J. R., Kandemir, B., Dickerson, C. E. & Bren, K. L. A cobalt metallopeptide electrocatalyst for the selective reduction of nitrite to ammonium. J. Am. Chem. Soc. 140, 16888–16892 (2018).
Park, J. et al. In situ electrochemical generation of nitric oxide for neuronal modulation. Nat. Nanotechnol. 15, 690–697 (2020).
Kim, D. H. et al. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 12, 1856 (2021).
Hao, Y.-C. et al. Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water. Nat. Catal. 2, 448–456 (2019).
Nakajima, K., Toda, H., Sakata, K. & Nishibayashi, Y. Ruthenium-catalysed oxidative conversion of ammonia into dinitrogen. Nat. Chem. 11, 702–709 (2019).
Kamiya, K. et al. Selective reduction of nitrate by a local cell catalyst composed of metal-doped covalent triazine frameworks. ACS Catal. 8, 2693–2698 (2018).
Chen, G.-F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Kim, J. E. et al. Electrochemical synthesis of glycine from oxalic acid and nitrate. Angew. Chem. Int. Ed. 60, 21943–21951 (2021).
Wu, Y. S., Jiang, Z., Lin, Z. C., Liang, Y. Y. & Wang, H. L. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 4, 725–730 (2021).
Lv, C. D. et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide. Nat. Sustain. 4, 868–876 (2021).
Koper, M. T. M. Volcano activity relationships for proton-coupled electron transfer reactions in electrocatalysis. Top. Catal. 58, 1153–1158 (2015).
Koper, M. T. M. Theory of multiple proton–electron transfer reactions and its implications for electrocatalysis. Chem. Sci. 4, 2710–2723 (2013).
He, D. et al. Selective electrocatalytic reduction of nitrite to dinitrogen based on decoupled proton-electron transfer. J. Am. Chem. Soc. 140, 2012–2015 (2018).
He, D. et al. Atomic-scale evidence for highly selective electrocatalytic N–N coupling on metallic MoS2. Proc. Natl Acad. Sci. USA 117, 31631–31638 (2020).
Liu, T. et al. Accelerating proton-coupled electron transfer of metal hydrides in catalyst model reactions. Nat. Chem. 10, 881–887 (2018).
Ooka, H., McGlynn, S. E. & Nakamura, R. Electrochemistry at deep‐sea hydrothermal vents: utilization of the thermodynamic driving force towards the autotrophic origin of life. ChemElectroChem 6, 1316–1323 (2019).
Liu, Q. et al. Gram-scale aqueous synthesis of stable few-layered 1T-MoS2: applications for visible-light-driven photocatalytic hydrogen evolution. Small 11, 5556–5564 (2015).
Yang, S. et al. Hierarchical 1T-MoS2 nanotubular structures for enhanced supercapacitive performance. J. Mater. Chem. A 5, 23704–23711 (2017).
Kwon, I. S. et al. Intercalation of aromatic amine for the 2H-1T′ phase transition of MoS2 by experiments and calculations. Nanoscale 10, 11349–11356 (2018).
Chou, S. S. et al. Understanding catalysis in a multiphasic two-dimensional transition metal dichalcogenide. Nat. Commun. 6, 8311 (2015).
Anderson, L. J., Richardson, D. J. & Butt, J. N. Catalytic protein film voltammetry from a respiratory nitrate reductase provides evidence for complex electrochemical modulation of enzyme activity. Biochemistry 40, 11294–11307 (2001).
Maia, L.B., Moura, I. & Moura, J.J.G. in Future Directions in Metalloprotein and Metalloenzyme Research, Vol. 33 (eds Hanson, G. & Berliner, L.) 55–101 (Springer, 2017).
Boussac, A. et al. The low spin-high spin equilibrium in the S2-state of the water oxidizing enzyme. Biochim. Biophys. Acta Bioenerg. 1859, 342–356 (2018).
Li, Y. et al. Enzyme mimetic active intermediates for nitrate reduction in neutral aqueous media. Angew. Chem. Int. Ed. 59, 9744–9750 (2020).
Glasser, N. R., Oyala, P. H., Osborne, T. H., Santini, J. M. & Newman, D. K. Structural and mechanistic analysis of the arsenate respiratory reductase provides insight into environmental arsenic transformations. Proc. Natl Acad. Sci. USA 115, E8614–E8623 (2018).
Tran, P. D. et al. Coordination polymer structure and revisited hydrogen evolution catalytic mechanism for amorphous molybdenum sulfide. Nat. Mater. 15, 640–646 (2016).
Andersen, S. Z. et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 570, 504–508 (2019).
Suryanto, B. H. R. et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2, 290–296 (2019).
Yang, H., Gandhi, H., Ostrom, N. E. & Hegg, E. L. Isotopic fractionation by a fungal P450 nitric oxide reductase during the production of N2O. Environ. Sci. Technol. 48, 10707–10715 (2014).
Stein, L. Y. & Yung, Y. L. Production, isotopic composition, and atmospheric fate of biologically produced nitrous oxide. Annu. Rev. Earth Planet. Sci. 31, 329–356 (2003).
Toyoda, S., Mutobe, H., Yamagishi, H., Yoshida, N. & Tanji, Y. Fractionation of N2O isotopomers during production by denitrifier. Soil Biol. Biochem. 37, 1535–1545 (2005).
Yoshida, N. & Toyoda, S. Constraining the atmospheric N2O budget from intramolecular site preference in N2O isotopomers. Nature 405, 330–334 (2000).
Maia, L. B. & Moura, J. J. How biology handles nitrite. Chem. Rev. 114, 5273–5357 (2014).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Acknowledgements
We thank H. Ofuchi at SPring-8 for his assistance with the XAFS measurements. R.N. was supported by a JSPS Grant-in-Aid for Scientific Research (number 26288092). H.O. was supported by Fusion Oriented Research for Disruptive Science and Technology program of Japan Science and Technology Agency (JST). Y.L. was supported by a JSPS Grant-in-Aid for Scientific Research (number 19K15671). N.Y. and S.T. were supported by a JSPS Kiban-S Grant-in-Aid (number 17H06105). S.H.K. was supported by the Creative Materials Discovery Program (NRF-2017M3D1A1039380). The synchrotron radiation experiments were performed at the BL14B2 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (proposal numbers 2021A1664, 2021B1920, 2022A1045 and 2022A1669).
Author information
Authors and Affiliations
Contributions
D. He and R.N. conceived and designed the experiments. D. He performed the experiments. H.O. performed the theoretical simulations. Y.L., N.Y. and S.T. performed the site-preference isotope measurements. Y.K., A.Y. and S.H.K. performed the EPR measurements. K.A. and D. Hashizume performed the XAFS measurements. D. He, H.O. and R.N. co-wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Bin Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, Tables 1–10, note 1 and references
Supplementary Data 1
The code for numerical simulation of the Faradaic efficiency.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, D., Ooka, H., Li, Y. et al. Regulation of the electrocatalytic nitrogen cycle based on sequential proton–electron transfer. Nat Catal 5, 798–806 (2022). https://doi.org/10.1038/s41929-022-00833-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00833-z
This article is cited by
-
Sustainable conversion of alkaline nitrate to ammonia at activities greater than 2 A cm−2
Nature Communications (2024)
-
Construction of electron rich Fe active sites by FeCu alloy anchoring on carbon nitride for photocatalytic nitrogen reduction
Rare Metals (2024)
-
Enhancing Hydrogen Diffusion in Catalytic Removal of Nitrate Using a Flow Reactor
Topics in Catalysis (2023)