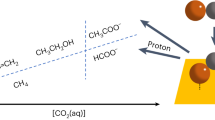
Abstract
Electrocatalytic conversion of carbon monoxide (CO) is being actively developed as a key component for tandem CO2 electrolysis. Great effort has been devoted to engineering CO reduction electrocatalysts for better multicarbon product selectivity. However, less work has focused on other performance parameters that are crucial for commercializing CO electrolysis, such as liquid product concentration and purity. Here, we present an internally coupled purification strategy to substantially improve the acetate concentration and purity in CO electrolysis. This strategy utilizes an alkaline-stable anion exchange membrane with high ethanol permeability and a selective ethanol partial oxidation anode to control the CO reduction product stream. We demonstrate stable 120-h continuous operation of the CO electrolyser at a current density of 200 mA cm−2 and a full-cell potential of <2.3 V, continuously producing a 1.9 M acetate product stream with a purity of 97.7%. The acetate stream was further improved to a concentration of 7.6 M at >99% purity by tuning the reaction conditions. Finally, a techno-economic analysis shows that a highly concentrated liquid product stream is essential to reduce the energy consumption of product separation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data is available from the authors upon reasonable request. Source data are provided with this paper.
References
Overa, S., Feric, T. G., Park, A. H. A. & Jiao, F. Tandem and hybrid processes for carbon dioxide utilization. Joule 5, 8–13 (2021).
Bushuyev, O. S. et al. What should we make with CO2 and how can we make it? Joule 2, 825–832 (2018).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Jeng, E. & Jiao, F. Investigation of CO2 single-pass conversion in a flow electrolyzer. React. Chem. Eng. 5, 1768–1775 (2020).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 10–12 (2020).
Cofell, E. R., Nwabara, U. O., Bhargava, S. S., Henckel, D. E. & Kenis, P. J. A. Investigation of electrolyte-dependent carbonate formation on gas diffusion electrodes for CO2 electrolysis. ACS Appl. Mater. Interfaces 13, 15132–15142 (2021).
Li, Y. C. et al. CO2 electroreduction from carbonate electrolyte. ACS Energy Lett. 4, 1427–1431 (2019).
Yan, Z., Hitt, J. L., Zeng, Z., Hickner, M. A. & Mallouk, T. E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Nat. Chem. 13, 33–40 (2021).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Cuellar, N. S. R., Scherer, C. & Ka, B. Two-step electrochemical reduction of CO2 towards multi-carbon products at high current densities. J. CO2 Util. 36, 263–275 (2020).
Ozden, A. et al. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 5, 706–710 (2021).
Ripatti, D. S., Veltman, T. R. & Kanan, M. W. Carbon monoxide gas diffusion electrolysis that produces concentrated C2 products with high single-pass conversion. Joule 3, 240–256 (2019).
Jouny, M., Hutchings, G. S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2, 1062–1070 (2019).
Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2, 423–430 (2019).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Zhu, P. et al. Direct and continuous generation of pure acetic acid solutions via electrocatalytic carbon monoxide reduction. Proc. Natl Acad. Sci. USA 118, e2010868118 (2021).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Shin, H., Kentaro, H. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
Zhu, P. & Wang, H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 4, 943–951 (2021).
Miller, M. & Bazylak, A. A review of polymer electrolyte membrane fuel cell stack testing. J. Power Sources 196, 601–613 (2011).
Marx, N., Boulon, L., Gustin, F., Hissel, D. & Agbossou, K. A review of multi-stack and modular fuel cell systems: interests, application areas and on-going research activities. Int. J. Hydrog. Energy 39, 12101–12111 (2014).
Selamet, Ö. F., Becerikli, F., Mat, M. D. & Kaplan, Y. Development and testing of a highly efficient proton exchange membrane (PEM) electrolyzer stack. Int. J. Hydrog. Energy 36, 11480–11487 (2011).
Noh, S., Jeon, J. Y., Adhikari, S., Kim, Y. S. & Bae, C. Molecular engineering of hydroxide conducting polymers for anion exchange membranes in electrochemical energy conversion technology. Acc. Chem. Res. 52, 2745–2755 (2019).
Sano, K. I., Uchida, H. & Wakabayashi, S. A new process for acetic acid production by direct oxidation of ethylene. Catal. Surv. Jpn 3, 55–60 (1999).
Etzi Coller Pascuzzi, M., Man, A. J. W., Goryachev, A., Hofmann, J. P. & Hensen, E. J. M. Investigation of the stability of NiFe-(oxy)hydroxide anodes in alkaline water electrolysis under industrially relevant conditions. Catal. Sci. Technol. 10, 5593–5601 (2020).
Scheres Firak, D., Rocha Ribeiro, R., de Liz, M. V. & Peralta-Zamora, P. Investigations on iron leaching from oxides and its relevance for radical generation during Fenton-like catalysis. Environ. Earth Sci. 77, 1–9 (2018).
Heenen, H. H. et al. Mechanism for acetate formation in CO(2) reduction on Cu: selectivity trends with pH and nanostructuring derive from mass transport. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv-2021-p3d4s (2021).
Dinh, C. T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Yuan, N., Jiang, Q., Li, J. & Tang, J. A review on non-noble metal based electrocatalysis for the oxygen evolution reaction. Arab. J. Chem. 13, 4294–4309 (2020).
Lu, X. & Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 6, 6616 (2015).
Lees, E. W., Mowbray, B. A. W., Parlane, F. G. L. & Berlinguetter, C. P. Gas diffusion electrodes and membranes for CO2 reduction electrolyzers. Nat. Rev. Mater. 7, 55–64 (2022).
Spurgeon, J. M. & Kumar, B. A comparative technoeconomic analysis of pathways for commercial electrochemical CO2 reduction to liquid products. Energy Environ. Sci. 11, 1536–1551 (2018).
Ramdin, M. et al. Electroreduction of CO2/CO to C2 products: process modeling, downstream separation, system integration, and economic analysis. Ind. Eng. Chem. Res. 60, 17862–17880 (2021).
Luc, W., Rosen, J. & Jiao, F. An Ir-based anode for a practical CO2 electrolyzer. Catal. Today 288, 79–84 (2017).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Acknowledgements
This material is based upon work supported by the US Department of Energy under Award Number DE-FE0031910.
Author information
Authors and Affiliations
Contributions
S.O. conducted the experiments, performed the data analysis and wrote the first draft of the manuscript. B.S.C. conducted the techno-economic analysis. B.S. and D.T. produced and conducted the analysis of the anion exchange membrane. B.H.K. performed the XPS measurements and analysed the XPS data. H.S. conducted the scanning electron microscopy measurements. C.B. supervised the synthesis of the anion exchange membranes. F.J. revised the manuscript and supervised the whole project. All authors commented on the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–5, Figs. 1–43 and Tables 1–8.
Source data
Source Data Fig. 1
Data for 120-h durability in Fig. 1b.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Overa, S., Crandall, B.S., Shrimant, B. et al. Enhancing acetate selectivity by coupling anodic oxidation to carbon monoxide electroreduction. Nat Catal 5, 738–745 (2022). https://doi.org/10.1038/s41929-022-00828-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00828-w
This article is cited by
-
Site-selective protonation enables efficient carbon monoxide electroreduction to acetate
Nature Communications (2024)
-
Durable CO2 conversion in the proton-exchange membrane system
Nature (2024)
-
Co-electrolysis of ethylene glycol and carbon dioxide for formate synthesis
Science China Chemistry (2024)
-
Scalable electrosynthesis of commodity chemicals from biomass by suppressing non-Faradaic transformations
Nature Communications (2023)
-
Mechanism-guided realization of selective carbon monoxide electroreduction to methanol
Nature Synthesis (2023)