Abstract
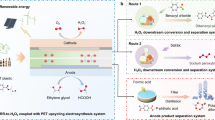
Electrochemical valorization of surplus biomass-derived feedstocks, such as glycerol, into high-value chemicals offers a sustainable route for utilization of biomass resources and decarbonization of chemical manufacturing; however, glycerol is typically valorized solely via anodic oxidation, with lower-value products such as hydrogen gas generated at the cathode. Here, we establish the efficient cathodic valorization of glycerol to the desirable C3 oxidation products via the electro-Fenton process at a stable NiSe2 cathode, built upon the theoretical understanding and experimental demonstration of the high selectivity and stability of NiSe2 toward acidic H2O2 electrosynthesis. A proof-of-concept linear paired electrochemical process for concurrent valorization of glycerol into the same oxidation products at both NiSe2 cathode and Pt anode achieves high selectivity for value-added C3 products and high glycerol conversion with little external energy input needed, when the electro-Fenton generation of hydroxyl radicals is carefully controlled. This conceptual strategy of linear pairing is generalizable for enabling atom-efficient electro-refinery of diverse biomass-derived feedstocks.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are presented in the article and Supplementary Information. Source data are provided with this paper. Any other relevant data are also available from the corresponding authors upon reasonable request.
References
Luna, P. D. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Tang, C., Zheng, Y., Jaroniec, M. & Qiao, S.-Z. Electrocatalytic refinery for sustainable production of fuels and chemicals. Angew. Chem. Int. Ed. Engl. 60, 19572–19590 (2021).
Lucas, F. W. S. et al. Electrochemical routes for the valorization of biomass-derived feedstocks: from chemistry to application. ACS Energy Lett. 6, 1205–1270 (2021).
Werpy, T. & Petersen, G. Top Value Added Chemicals from Biomass Technical Report (US Department of Energy, 2004); https://doi.org/10.2172/926125
Da Silva Ruy, A. D., Ferreira, A. L. F., Bresciani, A. É., de Brito Alves, R. M. & Pontes, L. A. M. in Biotechnological Applications of Biomass (eds Peixoto Basso, T. et al.) Ch. 11 (IntechOpen, 2021); https://www.intechopen.com/chapters/73542
Pagliaro, M., Ciriminna, R., Kimura, H., Rossi, M. & Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. Engl. 46, 4434–4440 (2007).
Katryniok, B. et al. Selective catalytic oxidation of glycerol: perspectives for high value chemicals. Green Chem. 13, 1960–1979 (2011).
Dodekatos, G., Schünemann, S. & Tüysüz, H. Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS Catal. 8, 6301–6333 (2018).
Simões, M., Baranton, S. & Coutanceau, C. Electrochemical valorisation of glycerol. ChemSusChem 5, 2106–2124 (2012).
Li, T. & Harrington, D. A. An overview of glycerol electrooxidation mechanisms on Pt, Pd and Au. ChemSusChem 14, 1472–1495 (2021).
Kwon, Y., Schouten, K. J. P. & Koper, M. T. M. Mechanism of the catalytic oxidation of glycerol on polycrystalline gold and platinum electrodes. ChemCatChem 3, 1176–1185 (2011).
Kwon, Y., Birdja, Y., Spanos, I., Rodriguez, P. & Koper, M. T. M. Highly selective electro-oxidation of glycerol to dihydroxyacetone on platinum in the presence of bismuth. ACS Catal. 2, 759–764 (2012).
Lee, S. et al. Highly selective transformation of glycerol to dihydroxyacetone without using oxidants by a PtSb/C-catalyzed electrooxidation process. Green Chem. 18, 2877–2887 (2016).
Fan, L. et al. Recent progress in electrocatalytic glycerol oxidation. Energy Technol. 9, 2000804 (2021).
Li, Y., Wei, X., Chen, L., Shi, J. & He, M. Nickel-molybdenum nitride nanoplate electrocatalysts for concurrent electrolytic hydrogen and formate productions. Nat. Commun. 10, 5335 (2019).
Han, X. et al. Electrocatalytic oxidation of glycerol to formic acid by CuCo2O4 spinel oxide nanostructure catalysts. ACS Catal. 10, 6741–6752 (2020).
Simões, M., Baranton, S. & Coutanceau, C. Electro-oxidation of glycerol at Pd based nano-catalysts for an application in alkaline fuel cells for chemicals and energy cogeneration. Appl. Catal. B 93, 354–362 (2010).
Chen, Y. X. et al. Nanotechnology makes biomass electrolysis more energy efficient than water electrolysis. Nat. Commun. 5, 4036 (2014).
Dagdougui, H., Sacile, R., Bersani, C. & Ouammi, A. in Hydrogen Infrastructure for Energy Applications (eds Dagdougui, H. et al.) Ch. 2 (Academic Press, 2018).
Liu, D. et al. Selective photoelectrochemical oxidation of glycerol to high value-added dihydroxyacetone. Nat. Commun. 10, 1779 (2019).
Verma, S., Lu, S. & Kenis, P. J. A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Nat. Energy 4, 466–474 (2019).
Yadegari, H. et al. Glycerol oxidation pairs with carbon monoxide reduction for low-voltage generation of C2 and C3 product streams. ACS Energy Lett. 6, 3538–3544 (2021).
Li, R., Xiang, K., Peng, Z., Zou, Y. & Wang, S. Recent advances on electrolysis for simultaneous generation of valuable chemicals at both anode and cathode. Adv. Energy Mater. 11, 2102292 (2021).
Aust, N. & Kirste, A. in Encyclopedia of Applied Electrochemistry (eds Kreysa, G. et al.) 1505–1510 (Springer, 2014).
Ibanez, J. G., Frontana-Uribe, B. A. & Vasquez-Medrano, R. Paired electrochemical processes: overview, systematization, selection criteria, design strategies, and projection. J. Mex. Chem. Soc. 60, 247–260 (2016).
Strehl, J., Abraham, M. L. & Hilt, G. Linear paired electrolysis—realising 200% current efficiency for stoichiometric transformations—the electrochemical bromination of alkenes. Angew. Chem. Int. Ed. Engl. 60, 9996–10000 (2021).
Yang, S. et al. Toward the decentralized electrochemical production of H2O2: a focus on the catalysis. ACS Catal. 8, 4064–4081 (2018).
Perry, S. C. et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 3, 442–458 (2019).
Brillas, E., Sirés, I. & Oturan, M. A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 109, 6570–6631 (2009).
Teong, S. P., Li, X. & Zhang, Y. Hydrogen peroxide as an oxidant in biomass-to-chemical processes of industrial interest. Green. Chem. 21, 5753–5780 (2019).
Moody, G. J. The action of fenton’s reagent on carbohydrates. Tetrahedron 19, 1705–1710 (1963).
Vitale, A. A., Bernatene, E. A., Vitale, M. G. & Pomilio, A. B. New insights of the Fenton reaction using glycerol as the experimental model. Effect of O2, inhibition by Mg2+, and oxidation state of Fe. J. Phys. Chem. A 120, 5435–5445 (2016).
Zeng, J. et al. Biomimetic Fenton-catalyzed lignin depolymerization to high-value aromatics and dicarboxylic acids. ChemSusChem 8, 861–871 (2015).
Sheng, H. et al. Electrocatalytic production of H2O2 by selective oxygen reduction using earth-abundant cobalt pyrite (CoS2). ACS Catal. 9, 8433–8442 (2019).
Sheng, H. et al. Stable and selective electrosynthesis of hydrogen peroxide and the electro-Fenton process on CoSe2 polymorph catalysts. Energy Environ. Sci. 13, 4189–4203 (2020).
Siahrostami, S. et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 12, 1137–1143 (2013).
Siahrostami, S. et al. A review on challenges and successes in atomic-scale design of catalysts for electrochemical synthesis of hydrogen peroxide. ACS Catal. 10, 7495–7511 (2020).
Pourbaix diagram. The Materials Project https://legacy.materialsproject.org/#apps/pourbaixdiagram/ (accessed December 2021).
Ryu, J. et al. Thermochemical aerobic oxidation catalysis in water can be analysed as two coupled electrochemical half-reactions. Nat. Catal. 4, 742–752 (2021).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal—amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Bahn, S. R. & Jacobsen, K. W. An object-oriented scripting interface to a legacy electronic structure code. Comput. Sci. Eng. 4, 56–66 (2002).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865; erratum Phys. Rev. Lett. 78, 1396 (1997).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Anisimov, V. I., Zaanen, J. & Andersen, O. K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 44, 943–954 (1991).
Wu, X. et al. Metal organic framework derived Fe-doped CoSe2 incorporated in nitrogen-doped carbon hybrid for efficient hydrogen evolution. ACS Sustain. Chem. Eng. 6, 8672–8678 (2018).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Mathew, K., Kolluru, V. S. C., Mula, S., Steinmann, S. N. & Hennig, R. G. Implicit self-consistent electrolyte model in plane-wave density-functional theory. J. Chem. Phys. 151, 234101 (2019).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Alchagirov, A. B., Perdew, J. P., Boettger, J. C., Albers, R. C. & Fiolhais, C. Energy and pressure versus volume: equations of state motivated by the stabilized jellium model. Phys. Rev. B 63, 224115 (2001).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Henkelman, G., Jóhannesson, G. & Jónsson, H. in Theoretical Methods in Condensed Phase Chemistry (eds. Schwartz, S. D.) 269–302 (Springer, 2002).
Henkelman, G. & Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 111, 7010–7022 (1999).
Heyden, A., Bell, A. T. & Keil, F. J. Efficient methods for finding transition states in chemical reactions: comparison of improved dimer method and partitioned rational function optimization method. J. Chem. Phys. 123, 224101 (2005).
Kästner, J. & Sherwood, P. Superlinearly converging dimer method for transition state search. J. Chem. Phys. 128, 014106 (2008).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Bureau, C. & Lécayon, G. On a modeling of voltage-application to metallic electrodes using density functional theory. J. Chem. Phys. 106, 8821–8829 (1997).
Letchworth-Weaver, K. & Arias, T. A. Joint density functional theory of the electrode-electrolyte interface: application to fixed electrode potentials, interfacial capacitances, and potentials of zero charge. Phys. Rev. B 86, 075140 (2012).
Duan, Z. & Henkelman, G. Theoretical resolution of the exceptional oxygen reduction activity of Au(100) in alkaline media. ACS Catal. 9, 5567–5573 (2019).
Abidi, N., Lim, K. R. G., Seh, Z. W. & Steinmann, S. N. Atomistic modeling of electrocatalysis: are we there yet? WIREs Comput. Mol. Sci. 11, e1499 (2021).
Hydrogen peroxide. NIST Chemistry WebBook https://webbook.nist.gov/cgi/cbook.cgi?ID=C7722841&Mask=10#Solubility (accessed December 2021).
Vinogradova, O., Krishnamurthy, D., Pande, V. & Viswanathan, V. Quantifying confidence in DFT-predicted surface Pourbaix diagrams of transition-metal electrode–electrolyte interfaces. Langmuir 34, 12259–12269 (2018).
Hansen, H. A., Rossmeisl, J. & Nørskov, J. K. Surface Pourbaix diagrams and oxygen reduction activity of Pt, Ag and Ni(111) surfaces studied by DFT. Phys. Chem. Chem. Phys. 10, 3722–3730 (2008).
Viswanathan, V., Hansen, H. A., Rossmeisl, J. & Nørskov, J. K. Unifying the 2e– and 4e– reduction of oxygen on metal surfaces. J. Phys. Chem. Lett. 3, 2948–2951 (2012).
Kulkarni, A., Siahrostami, S., Patel, A. & Nørskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Ford, D. C., Nilekar, A. U., Xu, Y. & Mavrikakis, M. Partial and complete reduction of O2 by hydrogen on transition metal surfaces. Surf. Sci. 604, 1565–1575 (2010).
Bhat, K. S., Barshilia, H. C. & Nagaraja, H. S. Porous nickel telluride nanostructures as bifunctional electrocatalyst towards hydrogen and oxygen evolution reaction. Int. J. Hydrog. Energy 42, 24645–24655 (2017).
Kolb, E. D. & Laudise, R. A. The solubility of trigonal Se in Na2S solutions and the hydrothermal growth of Se. J. Cryst. Growth 8, 191–196 (1971).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Lu, Z. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 1, 156–162 (2018).
Jiang, K. et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 10, 3997 (2019).
Acknowledgements
This research was supported by the National Science Foundation (NSF, grant no. CHE-1955074) for materials synthesis and electrochemical experiments (H.S., R.D.R. and S.J.) and for computational modelling (A.N.J., K.L. and J.R.S.). The calculations performed utilized computational resources from the Extreme Science and Engineering Discovery Environment supported by NSF grant no. TG-CHE120088. The Bruker AVANCE III 600 MHz NMR spectrometer was supported by National Institutes of Health grant no. S10 OK012245. The authors gratefully acknowledge use of the facilities and instrumentation at the UW-Madison Wisconsin Centers for Nanoscale Technology (wcnt.wisc.edu), partially supported by the NSF through the University of Wisconsin Materials Research Science and Engineering Center (no. DMR-1720415). We thank J. Lazarcik for help with gaining access to the ICP–MS spectrometer supported by the Water Science and Engineering Laboratory at UW-Madison. This research used resources of the APS, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. We thank Y. Ding for help with XAS experiments performed at APS Beamline 10-BM-B.
Author information
Authors and Affiliations
Contributions
H.S. and S.J. designed the experiments. H.S. carried out materials synthesis, materials characterization (with the help of R.D.R.), electrochemical measurements and product analyses of glycerol valorization (with the help of H.H.). A.N.J. and J.R.S. conceived computational modelling of the catalyst. A.N.J. and K.L. performed computational modelling. H.S. and S.J. wrote the manuscript, and all authors commented on it.
Corresponding authors
Ethics declarations
Competing interests
A provisional patent has been filed based on this work by some of the authors of this manuscript (H.S., A.N.J., R.D.R., K.L., J.R.S. and S.J.). The remaining author (H.H.) declares no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Chuang Peng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–29, Notes 1–6, Tables 1–4 and references.
Supplementary Data 1
Computational data.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
About this article
Cite this article
Sheng, H., Janes, A.N., Ross, R.D. et al. Linear paired electrochemical valorization of glycerol enabled by the electro-Fenton process using a stable NiSe2 cathode. Nat Catal 5, 716–725 (2022). https://doi.org/10.1038/s41929-022-00826-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00826-y
This article is cited by
-
Bias-free solar NH3 production by perovskite-based photocathode coupled to valorization of glycerol
Nature Catalysis (2024)
-
Electrochemical hydrogenation and oxidation of organic species involving water
Nature Reviews Chemistry (2024)
-
Constructing sulfur and oxygen super-coordinated main-group electrocatalysts for selective and cumulative H2O2 production
Nature Communications (2024)
-
Pulse potential mediated selectivity for the electrocatalytic oxidation of glycerol to glyceric acid
Nature Communications (2024)
-
Paired photoelectrochemical conversion of CO2/H2O and glycerol at high rate
Nature Catalysis (2024)