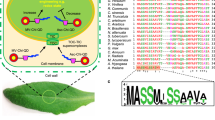
Abstract
Viral diseases of plants are associated with large health and economic costs. Antiviral agents developed for mammalian organisms have had limited success for plants, necessitating alternative strategies to address this biological and sustainability problem. Here we show that chiral 3 nm Cu1.96S nanoparticles can site-selectively cleave capsid in tobacco mosaic virus under sunlight. With d-penicillamine as surface ligands, the nanoparticles display high affinity to the Gln 99 to Ala 105 segment in the capsid via a network of supramolecular bonds and 3,000–10,000 times lower affinity to capsids of other viruses. Illumination with green light leads to polarization-dependent, protease-like hydrolysis of the amide bond between Asn 101 and Pro 102. Nanoparticles inhibited viral infectivity by 98.7% in protoplasts and 92.6% in plants while avoiding hypersensitive response and large environmental impact. These findings show that nanoparticles combining proteolytic activity due to metal ions and site selectivity due to nanoscale chirality can be used as effective antiviral agents.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. The simulation data of the optimized models of Fig. 4 and the raw data of Table 2 are provided at https://doi.org/10.6084/m9.figshare.20045621. Source data are provided with this paper.
References
Kah, M., Tufenkji, N. & White, J. C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 14, 532–540 (2019).
He, S. & Creasey Krainer, K. M. Pandemics of people and plants: which is the greater threat to food security? Mol. Plant 13, 933–934 (2020).
Jiang, G. et al. A rice NBS-ARC gene conferring quantitative resistance to bacterial blight is regulated by a pathogen effector-inducible miRNA. Mol. Plant 13, 1752–1767 (2020).
Vriet, C., Russinova, E. & Reuzeau, C. Boosting crop yields with plant steroids. Plant Cell 24, 842–857 (2012).
Hofmann, T. et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 1, 416–425 (2020).
Manfrin, A., Hanggli, A., van den Wildenberg, J. & McNeill, K. Substituent effects on the direct photolysis of benzotrifluoride derivatives. Environ. Sci. Technol. 54, 11109–11117 (2020).
Yuan, X. et al. Frequent gain and loss of resistance against tobacco mosaic virus in Nicotiana species. Mol. Plant 8, 1813–1815 (2015).
Pumplin, N. & Voinnet, O. RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nat. Rev. Microbiol. 11, 745–760 (2013).
Zhu, H., Li, C. & Gao, C. Applications of CRISPR-Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 21, 661–677 (2020).
Demirer, G. S. et al. Nanotechnology to advance CRISPR-Cas genetic engineering of plants. Nat. Nanotechnol. 16, 243–250 (2021).
Giraldo, J. P., Wu, H., Newkirk, G. M. & Kruss, S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 14, 541–553 (2019).
Lv, X. et al. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 35, 4195–4203 (2014).
Chariou, P. L. et al. Soil mobility of synthetic and virus-based model nanopesticides. Nat. Nanotechnol. 14, 712–718 (2019).
Jones, S. T. et al. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 6, eaax9318 (2020).
Bowman, M. C. et al. Inhibition of HIV fusion with multivalent gold nanoparticles. J. Am. Chem. Soc. 130, 6896–6897 (2008).
Kong, B. et al. Virucidal nano-perforator of viral membrane trapping viral RNAs in the endosome. Nat. Commun. 10, 185 (2019).
Wang, W. et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 15, 406–416 (2020).
Mitter, N. et al. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 3, 16207 (2017).
Torney, F., Trewyn, B. G., Lin, V. S. & Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2, 295–300 (2007).
Chung, Y. H., Beiss, V., Fiering, S. N. & Steinmetz, N. F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano 14, 12522–12537 (2020).
Chariou, P. L. & Steinmetz, N. F. Delivery of pesticides to plant parasitic nematodes using tobacco mild green mosaic virus as a nanocarrier. ACS Nano 11, 4719–4730 (2017).
Yang, T. & Duncan, T. V. Challenges and potential solutions for nanosensors intended for use with foods. Nat. Nanotechnol. 16, 251–265 (2021).
Kotov, N. A. Chemistry. Inorganic nanoparticles as protein mimics. Science 330, 188–189 (2010).
Lauster, D. et al. Phage capsid nanoparticles with defined ligand arrangement block influenza virus entry. Nat. Nanotechnol. 15, 373–379 (2020).
Kwon, P. S. et al. Designer DNA architecture offers precise and multivalent spatial pattern-recognition for viral sensing and inhibition. Nat. Chem. 12, 26–35 (2020).
Vigant, F., Santos, N. C. & Lee, B. Broad-spectrum antivirals against viral fusion. Nat. Rev. Microbiol. 13, 426–437 (2015).
Cagno, V. et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 17, 195–203 (2018).
Xu, L. et al. Enantiomer-dependent immunological response to chiral nanoparticles. Nature 601, 366–373 (2022).
Dong, J. et al. Free-standing homochiral 2D monolayers by exfoliation of molecular crystals. Nature 602, 606–611 (2022).
Yan, J. et al. Self-assembly of chiral nanoparticles into semiconductor helices with tunable near-infrared optical activity. Chem. Mater. 32, 476–488 (2019).
Yang, M. et al. Self-assembly of nanoparticles into biomimetic capsid-like nanoshells. Nat. Chem. 9, 287–294 (2017).
Zhang, Q. et al. Unraveling the origin of chirality from plasmonic nanoparticle-protein complexes. Science 365, 1475–1478 (2019).
Sun, M. et al. Site-selective photoinduced cleavage and profiling of DNA by chiral semiconductor nanoparticles. Nat. Chem. 10, 821–830 (2018).
Hou, K. et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 11, 4790 (2020).
Ge, P. & Zhou, Z. H. Hydrogen-bonding networks and RNA bases revealed by cryo electron microscopy suggest a triggering mechanism for calcium switches. Proc. Natl Acad. Sci. USA 108, 9637–9642 (2011).
Zhang, H., Zhao, J., Liu, S., Zhang, D. P. & Liu, Y. Tm-22 confers different resistance responses against tobacco mosaic virus dependent on its expression level. Mol. Plant 6, 971–974 (2013).
Ge, P. & Zhou, Z. H. Hydrogen-bonding networks and RNA bases revealed by cryo electron microscopy suggest a triggering mechanism for calcium switches. Proc. Natl Acad. Sci. USA 108, 9637–9642 (2011).
Kim, J. Y. et al. Assembly of gold nanoparticles into chiral superstructures driven by circularly polarized light. J. Am. Chem. Soc. 141, 11739–11744 (2019).
Qu, A. et al. Stimulation of neural stem cell differentiation by circularly polarized light transduced by chiral nanoassemblies. Nat. Biomed. Eng. 5, 103–113 (2021).
Yeom, J. et al. Chiromagnetic nanoparticles and gels. Science 359, 309–314 (2018).
Moloney, M. P., Gun’ko, Y. K. & Kelly, J. M. Chiral highly luminescent CdS quantum dots. Chem. Commun. (Camb.) 38, 3900–3902 (2007).
Sperling, R. A., Rivera Gil, P., Zhang, F., Zanella, M. & Parak, W. J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 37, 1896–1908 (2008).
Liu, J. et al. Luminescent gold nanoparticles with size-independent emission. Angew. Chem. Int. Ed. 55, 8894–8898 (2016).
Sharma, P., Jha, A. B., Dubey, R. S. & Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1–26 (2012).
Yin, J. J. et al. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes—generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharmacol. 263, 81–88 (2012).
Pospisil, P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front Plant Sci. 7, 1950–1962 (2016).
Jones, B. J., Vergne, M. J., Bunk, D. M., Locascio, L. E. & Hayes, M. A. Cleavage of peptides and proteins using light-generated radicals from titanium dioxide. Anal. Chem. 79, 1327–1332 (2007).
Ludwig, J. et al. Ultrafast hole trapping and relaxation dynamics in p-type CuS nanodisks. J. Phys. Chem. Lett. 6, 2671–2675 (2015).
Gilmanshin, R., Williams, S., Callender, R. H., Woodruff, W. H. & Dyer, R. B. Fast events in protein folding: relaxation dynamics of secondary and tertiary structure in native apomyoglobin. Proc. Natl Acad. Sci. USA 94, 3709–3713 (1997).
Dolan, E. A., Yelle, R. B., Beck, B. W., Fischer, J. T. & Ichiye, T. Protein control of electron transfer rates via polarization: molecular dynamics studies of rubredoxin. Biophys. J. 86, 2030–2036 (2004).
Li, L., Li, C., Zhang, Z. & Alexov, E. On the dielectric “constant” of proteins: smooth dielectric function for macromolecular modeling and its implementation in DelPhi. J. Chem. Theory Comput. 9, 2126–2136 (2013).
Amin, M. & Kupper, J. Variations in proteins dielectric constants. ChemistryOpen 9, 691–694 (2020).
Ly, H. G. T. et al. Superactivity of MOF-808 toward peptide bond hydrolysis. J. Am. Chem. Soc. 140, 6325–6335 (2018).
El-Shetehy, M. et al. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 16, 344–353 (2021).
Hu, P. et al. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano 14, 7970–7986 (2020).
Guo, W., Lu, X., Liu, B., Yan, H. & Feng, J. Anti-TMV activity and mode of action of three alkaloids isolated from Chelidonium majus. Pest Manag Sci. 77, 510–517 (2021).
Adeel, M. et al. Carbon-based nanomaterials suppress tobacco mosaic virus (TMV) infection and induce resistance in Nicotiana benthamiana. J. Hazard. Mater. 404, 124167 (2021).
Gilbertson, L. M. et al. Guiding the design space for nanotechnology to advance sustainable crop production. Nat. Nanotechnol. 15, 801–810 (2020).
Kah, M., Kookana, R. S., Gogos, A. & Bucheli, T. D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 13, 677–684 (2018).
Wang, L. et al. Arabinogalactan protein–rare earth element complexes activate plant endocytosis. Proc. Natl Acad. Sci. USA 116, 14349–14357 (2019).
Wong, M. H. et al. Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat. Mater. 16, 264–272 (2017).
Dousset, S. et al. Facilitated transport of diuron and glyphosate in high copper vineyard soils. Environ. Sci. Technol. 41, 8056–8061 (2007).
Ma, G. X. et al. Self-assembly of copper sulfide nanoparticles into nanoribbons with continuous crystallinity. ACS Nano 7, 9010–9018 (2013).
Zhang, J., Zhou, K., Zhang, Y., Du, M. & Wang, Q. Precise self-assembly of nanoparticles into ordered nanoarchitectures directed by tobacco mosaic virus coat protein. Adv. Mater. 31, e1901485 (2019).
King, M. A. Detection of dead cells and measurement of cell killing by flow cytometry. J. Immunol. Methods 243, 155–166 (2000).
Gan, H. et al. Dynamics of 5-hydroxymethylcytosine during mouse spermatogenesis. Nat. Commun. 4, 1995 (2013).
Acknowledgements
This work is financially supported by the National Key R&D Program (2021YFA1200300), and is also supported by the National Natural Science Foundation of China (32071400, 21925402, 21977038). N.A.K is grateful to NSF 1463474 and NSF 1566460 for support. We are grateful to the Brazilian funding agencies CAPES (Finance Code 001), CNPq (process 311353/2019-3) and FAPESP (processes 2012/15147-4 and 2013/07296-2) for financial support and the HPC resources provided by the SDumont supercomputer at the National Laboratory for Scientific Computing (LNCC/MCTI, Brazil, http://sdumont.lncc.br) and by the Cloud@UFSCar.
Author information
Authors and Affiliations
Contributions
H.K., N.A.K. and C.X. conceived the project and designed the experiments. J.Y. was responsible for the antiviral experiments on tobacco and plant cells. R.G. was responsible for the synthesis and characterization of the chiral NPs and studied their photoinduced cleavage properties. R.G., L.X. and M.S. carried out the CD, western blot, XPS, X-ray diffraction, LC–MS–MS and ROS experiments. M.X. performed the test on antiviral activity in TMV plants. C.H. and X.G. helped to synthesis, characterize and analyse the mechanism. F.M.C., X.Z., P.K. and A.F.M. performed the MD, QM and DFT simulations, and analysed the results. H.K. and N.A.K. conceptualized the work. C.X. supervised the study. H.K., N.A.K. and C.X. analysed and discussed the results and wrote the manuscript
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Fabienne Schwab, Lixia Zhao, Vladimir Lobaskin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–42, Tables 1–6, notes 1–14 and references.
Supplementary Video 1
Isosurfaces for average electrostatic polarizations of D-NPs and L-NPs interacting with multiple QANPTTA segments of CP monomer in TMV capsid being illuminated with the light in the spectral window from 300 to 1000 nm.
Supplementary Video 2
Polarization maps according to the change in the electronic population for Ala100-Asn101-Pro102-Thr103 fragment (ANPT) interacting with D-NP, upon excitation at 533-534 nm.
Supplementary Video 3
Polarization maps according to the change in the electronic population for Ala100-Asn101-Pro102-Thr103 fragment (ANPT) interacting with L-NP, upon excitation at 533-534 nm.
Source data
Source Data Fig. 5
Statistical Source Data
Source Data Table 1
Statistical Source Data
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, R., Xu, L., Sun, M. et al. Site-selective proteolytic cleavage of plant viruses by photoactive chiral nanoparticles. Nat Catal 5, 694–707 (2022). https://doi.org/10.1038/s41929-022-00823-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00823-1
This article is cited by
-
Regulation of biological processes by intrinsically chiral engineered materials
Nature Reviews Materials (2023)
-
Chiral nanoparticle-remodeled gut microbiota alleviates neurodegeneration via the gut–brain axis
Nature Aging (2023)
-
Chiral nanomaterial-enabled bactericide and nanopesticide for sustainable agriculture and food safety
Journal of Nanoparticle Research (2023)
-
Chiral inorganic nanomaterials: Harnessing chirality-dependent interactions with living entities for biomedical applications
Nano Research (2023)