Abstract
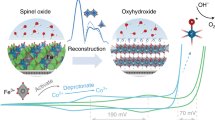
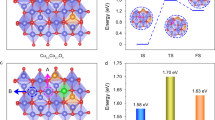
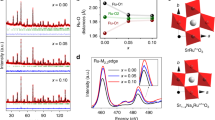
Active and stable electrocatalysts for the oxygen evolution reaction are required to produce hydrogen from water using renewable electricity. Here we report that incorporating Mn into the spinel lattice of Co3O4 can extend the catalyst lifetime in acid by two orders of magnitude while maintaining the activity. The activation barrier of the obtained spinel Co2MnO4 is comparable to that of state-of-the-art iridium oxides, most probably due to the ideal binding energies of the oxygen evolution reaction intermediates, as shown using density functional theory calculations. The calculations also show that the thermodynamic landscape of Co2MnO4 suppresses dissolution, which results in a lifetime of over 2 months (1,500 hours) at 200 mA cm−2geo at pH 1. As the lifetimes of other 3d metal oxygen evolution catalysts are in the order of days and weeks, despite current densities being lower by an order of magnitude, our results are an important step towards the realization of noble-metal-free water electrolysers.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. The atomic coordinates of the computational models are given in Supplementary Data 1. The structural parameters derived from the Rietveld refinement are given in Supplementary Data 2–4 for Co2MnO4 before electrolysis and after electrolysis for 4 h and 23 h, respectively. The structures are also available from the Cambridge Crystal Structure Data Centre (https://www.ccdc.cam.ac.uk/) with the deposition codes 2098166, 2098164 and 2098165 for Co2MnO4 before the electrolysis and after the electrolysis at 4 and 23 h, respectively.
References
Smalley, R. E. Future global energy prosperity: the terawatt challenge. MRS Bull. 30, 412–417 (2011).
Kibsgaard, J. & Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 4, 430–433 (2019).
Lewinski, K. A., Van der Vliet, D. & Luopa, S. M. NSTF advances for PEM electrolysis–the effect of alloying on activity of NSTF electrolyzer catalysts and performance of NSTF based PEM electrolyzers. ECS Trans. 69, 893–917 (2015).
Marshall, A. T., Sunde, S., Tsypkin, M. & Tunold, R. Performance of a PEM water electrolysis cell using IrxRuyTazO2 electrocatalysts for the oxygen evolution electrode. Int. J. Hydrogen Energy 32, 2320–2324 (2007).
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014 (2016).
Vesborg, P. C. K. & Jaramillo, T. F. Addressing the terawatt challenge: scalability in the supply of chemical elements for renewable energy. RSC Adv. 2, 7933–7947 (2012).
Cowley, A. PGM Market Report May 2020 (Johnson Matthey, 2020); https://matthey.com/en/news/2020/pgm-market-report-may-2020
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Blasco-Ahicart, M., Soriano-López, J., Carbó, J. J., Poblet, J. M. & Galan-Mascaros, J. R. Polyoxometalate electrocatalysts based on Earth-abundant metals for efficient water oxidation in acidic media. Nat. Chem. 10, 24–30 (2017).
Jiao, F. & Frei, H. Nanostructured cobalt oxide clusters in mesoporous silica as efficient oxygen-evolving catalysts. Angew. Chem. Int. Ed. 48, 1841–1844 (2009).
Mondschein, J. S. et al. Crystalline cobalt oxide films for sustained electrocatalytic oxygen evolution under strongly acidic conditions. Chem. Mater. 29, 950–957 (2017).
Han, L. et al. Enhanced activity and acid pH stability of Prussian blue-type oxygen evolution electrocatalysts processed by chemical etching. J. Am. Chem. Soc. 138, 16037–16045 (2016).
Huynh, M., Ozel, T., Liu, C., Lau, E. C. & Nocera, D. G. Design of template-stabilized active and Earth-abundant oxygen evolution catalysts in acid. Chem. Sci. 8, 4779–4794 (2017).
Kwong, W. L., Lee, C. C., Shchukarev, A. & Messinger, J. Cobalt-doped hematite thin films for electrocatalytic water oxidation in highly acidic media. Chem. Commun. 55, 5017–5020 (2019).
Anantharaj, S., Karthick, K. & Kundu, S. Spinel cobalt titanium binary oxide as an all-non-precious water oxidation electrocatalyst in acid. Inorg. Chem. 58, 8570–8576 (2019).
Chatti, M. et al. Intrinsically stable in situ generated electrocatalyst for long-term oxidation of acidic water at up to 80 °C. Nat. Catal. 2, 457–465 (2019).
Zhou, L. et al. Rutile alloys in the Mn–Sb–O system stabilize Mn3+ to enable oxygen evolution in strong acid. ACS Catal. 8, 10938–10948 (2018).
Moreno-Hernandez, I. A. et al. Crystalline nickel manganese antimonate as a stable water-oxidation catalyst in aqueous 1.0 M H2SO4. Energy Environ. Sci. 10, 2103–2108 (2017).
Ghadge, S. D. et al. Influence of defects on activity-stability of Cu1.5Mn1.5O4 for acid-mediated oxygen evolution reaction. J. Electrochem. Soc. 167, 144511 (2020).
Frydendal, R., Paoli, E. A., Chorkendorff, I., Rossmeisl, J. & Stephens, I. E. L. Toward an active and stable catalyst for oxygen evolution in acidic media: Ti-stabilized MnO2. Adv. Energy Mater. 5, 1500991 (2015).
Mondschein, J. S. et al. Intermetallic Ni2Ta electrocatalyst for the oxygen evolution reaction in highly acidic electrolytes. Inorg. Chem. 57, 6010–6015 (2018).
Schäfer, H. et al. Steel-based electrocatalysts for efficient and durable oxygen evolution in acidic media. Catal. Sci. Technol. 8, 2104–2116 (2018).
Hu, F. et al. Amorphous metallic NiFeP: a conductive bulk material achieving high activity for oxygen evolution reaction in both alkaline and acidic media. Adv. Mater. 29, 1606570 (2017).
Wu, J. et al. Exfoliated 2D transition metal disulfides for enhanced electrocatalysis of oxygen evolution reaction in acidic medium. Adv. Mater. Inter. 3, 1500669 (2016).
Patel, P. P. et al. Noble metal-free bifunctional oxygen evolution and oxygen reduction acidic media electro-catalysts. Sci Rep. 6, 28367 (2016).
Huynh, M., Bediako, D. K. & Nocera, D. G. A functionally stable manganese oxide oxygen evolution catalyst in acid. J. Am. Chem. Soc. 136, 6002–6010 (2014).
Li, A. et al. Stable potential windows for long-term electrocatalysis by manganese oxides under acidic conditions. Angew. Chem. Int. Ed. 58, 5054–5058 (2019).
McCrory, C. C. L., Jung, S., Peters, J. C. & Jaramillo, T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135, 16977–16987 (2013).
Forsythe, R. C. & Müller, A. M. Quo vadis water oxidation? Catal. Today https://doi.org/10.1016/j.cattod.2020.06.011 (in the press).
Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions (Pergamon, 1966).
Rios, E., Chartier, P. & Gautier, J. L. Oxygen evolution electrocatalysis in alkaline medium at thin MnxCo3–xO4 (0 ≤ x ≤ 1) spinel films on glass/SnO2: F prepared by spray pyrolysis. Solid State Sci. 1, 267–277 (1999).
Rietveld, H. M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 2, 65–71 (1969).
Navrotsky, A. & Kleppa, O. J. The thermodynamics of cation distributions in simple spinels. J. Inorg. Nucl. Chem. 29, 2701–2714 (1967).
Ayers, K. et al. Perspectives on low-temperature electrolysis and potential for renewable hydrogen at scale. Annu. Rev. Chem. Biomol. Eng. 10, 219–239 (2019).
Vargas-Barbosa, N. M. Insights into Electrochemical and Photoelectrochemical Water-Splitting (Pennsylvania State Univ., 2015).
Nurlaela, E., Shinagawa, T., Qureshi, M., Dhawale, D. S. & Takanabe, K. Temperature dependence of electrocatalytic and photocatalytic oxygen evolution reaction rates using NiFe oxide. ACS Catal. 6, 1713–1722 (2016).
Guo, C. et al. Toward computational design of chemical reactions with reaction phase diagram. Wiley Interdiscip. Rev. Comput. Mol. Sci. 11, e1514 (2021).
Lankauf, K. et al. MnxCo3–xO4 spinel oxides as efficient oxygen evolution reaction catalysts in alkaline media. Int. J. Hydrogen Energy 45, 14867–14879 (2020).
Chan, K. & Nørskov, J. K. Electrochemical barriers made simple. J. Phys. Chem. Lett. 6, 2663–2668 (2015).
Li, H., Guo, C., Fu, Q. & Xiao, J. Toward fundamentals of confined electrocatalysis in nanoscale reactors. J. Phys. Chem. Lett. 10, 533–539 (2019).
Campbell, C. T. The degree of rate control: a powerful tool for catalysis research. ACS Catal. 7, 2770–2779 (2017).
Guo, C., Mao, Y., Yao, Z., Chen, J. & Hu, P. Examination of the key issues in microkinetics: CO oxidation on Rh(111). J. Catal. 379, 52–59 (2019).
Xiao, J., Kuc, A., Frauenheim, T. & Heine, T. Stabilization mechanism of ZnO nanoparticles by Fe doping. Phys. Rev. Lett. 112, 106102 (2014).
Kato, K. & Tanaka, H. Visualizing charge densities and electrostatic potentials in materials by synchrotron X-ray powder diffraction. Adv. Phys. X 1, 55–80 (2016).
Kato, K., Tanaka, Y., Yamauchi, M., Ohara, K. & Hatsui, T. A statistical approach to correct X-ray response non-uniformity in microstrip detectors for high-accuracy and high-resolution total-scattering measurements. J. Synchrotron Radiat. 26, 762–773 (2019).
Izumi, F. & Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 130, 15–20 (2007).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Zhang, Y. & Yang, W. Comment on “Generalized gradient approximation made simple”. Phys. Rev. Lett. 80, 890–890 (1998).
Methfessel, M. & Paxton, A. T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 40, 3616–3621 (1989).
Zasada, F. et al. Surface structure and morphology of M[CoM′]O4 (M = Mg, Zn, Fe, Co and M′ = Ni, Al, Mn, Co) spinel nanocrystals—DFT+U and TEM screening investigations. J. Phys. Chem. 118, 19085–19097 (2014).
Wan, H., Østergaard, T. M., Arnarson, L. & Rossmeisl, J. Climbing the 3D volcano for the oxygen reduction reaction using porphyrin motifs. ACS Sustain. Chem. Eng. 7, 611–617 (2019).
Acknowledgements
We thank T. Kikitsu and K. Takahashi at RIKEN for assistance with TEM and ICP-MS measurements and analysis. The synchrotron radiation experiments were performed at the BL14B2 (XAFS) and BL44B2 (SR-PXRD) of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (proposal no. 2021A2013) and RIKEN (proposal no. 20210064), respectively. The XAFS spectrum of LiMn2O4 was utilized by the SPring-8 BL14B2 XAFS database. This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) and JSPS Grant-in-Aid for Scientific Research (no. 18H02070). H.H. also thanks the National Key R&D Program of China (no. 2017YFA0204804) and the National Natural Science Foundation of China (no. 21761142018). J.X. acknowledges the financial support from the National Key R&D Program of China (No. 2021YFA1500702), the DNL Cooperation Fund, CAS (no. DNL202003), the National Natural Science Foundation of China (22172156, 91845103, 91945302 and 21802124), the Strategic Priority Research Program of Chinese Academy of Sciences (no. XDB36030200), the funding support by the LiaoNing Revitalization Talents Program (XLYC1907099) and the State Key Laboratory of Catalysis in DICP (no. N-19-13).
Author information
Authors and Affiliations
Contributions
A.L., S.K. and R.N. conceived and designed the experiments; S.K., A.L., Q.J. and K.A. performed the experiments; C.G. and J.X. performed the DFT calculation; S.K., A.L., H.O., K.A., D.H. and R.N. analysed the data; A.L., S.K., C.G., H.O., H.H., J.X. and R.N. co-wrote the paper. All the authors discussed the results and critically reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Jongwoo Lim, Nan Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–59, Tables 1–24, Notes 1–3 and references.
Supplementary Video 1
Bubble generation from a Co2MnO4 catalyst deposited on an FTO substrate at room temperature (25 °C) at current densities of 20 mA cm−2geo, 50 mA cm−2geo, 100 mA cm−2geo, 200 mA cm−2geo, 500 mA cm−2geo and 1,000 mA cm−2geo, respectively.
Supplementary Data 1
The atomic coordinates of the computational models.
Supplementary Data 2
Crystallographic data of Co2MnO4 before electrolysis derived from Rietveld refinement.
Supplementary Data 3
Crystallographic data of Co2MnO4 after electrolysis at 100 mA cm−2geo (pH 1 H2SO4, 25 °C) for 4 h derived from Rietveld refinement.
Supplementary Data 4
Crystallographic data of Co2MnO4 after electrolysis at 100 mA cm−2geo (pH 1 H2SO4, 25 °C) for 23 h derived from Rietveld refinement.
Rights and permissions
About this article
Cite this article
Li, A., Kong, S., Guo, C. et al. Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid. Nat Catal 5, 109–118 (2022). https://doi.org/10.1038/s41929-021-00732-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00732-9
This article is cited by
-
Facilitating alkaline hydrogen evolution reaction on the hetero-interfaced Ru/RuO2 through Pt single atoms doping
Nature Communications (2024)
-
Surface oxidation/spin state determines oxygen evolution reaction activity of cobalt-based catalysts in acidic environment
Nature Communications (2024)
-
Acid-stable manganese oxides for proton exchange membrane water electrolysis
Nature Catalysis (2024)
-
Recent progress of cobalt-based electrocatalysts for water splitting: Electron modulation, surface reconstitution, and applications
Nano Research (2024)
-
Modulation in the electronic structure of Ir-rich shell on AuIr solid solution as OER electrocatalyst for PEM electrolyzer
Journal of Applied Electrochemistry (2024)