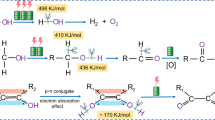
Abstract
Hydrogen production through water electrolysis is of considerable interest for converting the intermittent electricity generated by renewable energy sources into storable chemical energy, but the typical water electrolysis process requires a high working voltage (>1.23 V) and produces oxygen at the anode in addition to hydrogen at the cathode. Here we report a hydrogen production system that combines anodic and cathodic H2 production from low-potential aldehyde oxidation and the hydrogen evolution reaction, respectively, at a low voltage of ~0.1 V. Unlike conventional aldehyde electrooxidation, in which the hydrogen atom of the aldehyde group is oxidized into H2O at high potentials, the low-potential aldehyde oxidation enables the hydrogen atom to recombine into H2 gas. The assembled electrolyser requires an electricity input of only ~0.35 kWh per m3 of H2, in contrast to the ~5 kWh per m3 of H2 required for conventional water electrolysis. This study provides a promising avenue for the safe, efficient and scalable production of high-purity hydrogen.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are included in the published article and its Supplementary Information. All other data are available from the authors upon reasonable request. Source data are provided with this paper.
References
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017).
Tian, X. L. et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 366, 850–856 (2019).
Gong, K. P., Du, F., Xia, Z. H., Durstock, M. & Dai, L. M. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323, 760–764 (2009).
Nikolaidis, P. & Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 67, 597–611 (2017).
Lin, F. et al. Electrocatalytic hydrogen evolution of ultrathin Co-Mo5N6 heterojunction with interfacial electron redistribution. Adv. Energy Mater. 10, 2002176 (2020).
Wu, L. et al. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Adv. Funct. Mater. 31, 2006484 (2020).
Mahmood, J. et al. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 12, 441–446 (2017).
Li, H. et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 15, 48–53 (2016).
Zhang, B. et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat. Catal. 3, 985–992 (2020).
Kuai, C. et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts. Nat. Catal. 3, 743–753 (2020).
Oener, S. Z., Foster, M. J. & Boettcher, S. W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 369, 1099–1103 (2020).
Rausch, B., Symes, M. D., Chisholm, G. & Cronin, L. Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting. Science 345, 1326–1330 (2014).
You, B., Han, G. Q. & Sun, Y. J. Electrocatalytic and photocatalytic hydrogen evolution integrated with organic oxidation. Chem. Commun. 54, 5943–5955 (2018).
Chen, L., Dong, X. L., Wang, Y. G. & Xia, Y. Y. Separating hydrogen and oxygen evolution in alkaline water electrolysis using nickel hydroxide. Nat. Commun. 7, 11741 (2016).
Chen, G. F., Luo, Y. R., Ding, L. X. & Wang, H. H. Low-voltage electrolytic hydrogen production derived from efficient water and ethanol oxidation on fluorine-modified FeOOH anode. ACS Catal. 8, 526–530 (2018).
Chen, Y. X. et al. Nanotechnology makes biomass electrolysis more energy efficient than water electrolysis. Nat. Commun. 5, 4036 (2014).
You, B., Liu, X., Liu, X. & Sun, Y. Efficient H2 evolution coupled with oxidative refining of alcohols via a hierarchically porous nickel bifunctional electrocatalyst. ACS Catal. 7, 4564–4570 (2017).
Zheng, J. et al. Hierarchical porous NC@CuCo nitride nanosheet networks: highly efficient bifunctional electrocatalyst for overall water splitting and selective electrooxidation of benzyl alcohol. Adv. Funct. Mater. 27, 1704169 (2017).
Huang, H. et al. Ni, Co hydroxide triggers electrocatalytic production of high-purity benzoic acid over 400 mA cm−2. Energy Environ. Sci. 13, 4990–4999 (2020).
Chen, S., Duan, J. J., Vasileff, A. & Qiao, S. Z. Size fractionation of two-dimensional sub-nanometer thin manganese dioxide crystals towards superior urea electrocatalytic conversion. Angew. Chem. Int. Ed. 55, 3804–3808 (2016).
Liu, W. J. et al. Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat. Commun. 11, 265 (2020).
Du, P. Y., Zhang, J. J., Liu, Y. H. & Huang, M. H. Hydrogen generation from catalytic glucose oxidation by Fe-based electrocatalysts. Electrochem. Commun. 83, 11–15 (2017).
Jiang, N. et al. Electrocatalysis of furfural oxidation coupled with H2 evolution via nickel-based electrocatalysts in water. ChemNanoMat 3, 491–495 (2017).
Jiang, N., You, B., Boonstra, R., Rodriguez, I. M. T. & Sun, Y. J. Integrating electrocatalytic 5-hydroxymethylfurfural oxidation and hydrogen production via Co-P-derived electrocatalysts. ACS Energy Lett. 1, 386–390 (2016).
You, B., Jiang, N., Liu, X. & Sun, Y. J. Simultaneous H2 generation and biomass upgrading in water by an efficient noble-metal-free bifunctional electrocatalyst. Angew. Chem. Int. Ed. 55, 9913–9917 (2016).
You, B., Liu, X., Jiang, N. & Sun, Y. J. A general strategy for decoupled hydrogen production from water splitting by integrating oxidative biomass valorization. J. Am. Chem. Soc. 138, 13639–13646 (2016).
Zhang, N. N. et al. Electrochemical oxidation of 5-hydroxymethylfurfural on nickel nitride/carbon nanosheets: reaction pathway determined by in situ sum frequency generation vibrational spectroscopy. Angew. Chem. Int. Ed. 58, 15895–15903 (2019).
Zhou, B. et al. Platinum modulates redox properties and 5-hydroxymethylfurfural adsorption kinetics of Ni(OH)2 for biomass upgrading. Angew. Chem. Int. Ed. 60, 22908–22914 (2021).
Lu, Y. et al. Identifying the geometric site dependence of spinel oxides for the electrooxidation of 5-hydroxymethylfurfural. Angew. Chem. Int. Ed. 59, 19215–19221 (2020).
Lu, Y. et al. Tuning the selective adsorption site of biomass on Co3O4 by Ir single atoms for electrosynthesis. Adv. Mater. 33, 2007056 (2021).
Nam, D.-H., Taitt, B. J. & Choi, K.-S. Copper-based catalytic anodes to produce 2,5-furandicarboxylic acid, a biomass-derived alternative to terephthalic acid. ACS Catal. 8, 1197–1206 (2018).
Kapoor, S., Barnabas, F. A., Sauer, M. C., Meisel, D. & Jonah, C. D. Kinetics of hydrogen formation from formaldehyde in basic aqueous solutions. J. Phys. Chem. 99, 6857–6863 (1995).
Bi, Y. & Lu, G. Nano-Cu catalyze hydrogen production from formaldehyde solution at room temperature. Int. J. Hydrogen Energy 33, 2225–2232 (2008).
Gao, S. et al. Immobilizing AgPd alloy on Vulcan XC-72 carbon: a novel catalyst for highly efficient hydrogen generation from formaldehyde aqueous solution. RSC Adv. 6, 105638–105643 (2016).
Pan, X. et al. A novel biomass assisted synthesis of Au-SrTiO3 as a catalyst for direct hydrogen generation from formaldehyde aqueous solution at low temperature. Int. J. Hydrogen Energy 40, 1752–1759 (2015).
Hu, H., Jiao, Z., Ye, J., Lu, G. & Bi, Y. Highly efficient hydrogen production from alkaline aldehyde solutions facilitated by palladium nanotubes. Nano Energy 8, 103–109 (2014).
Kapoor, S. & Naumov, S. On the origin of hydrogen in the formaldehyde reaction in alkaline solution. Chem. Phys. Lett. 387, 322–326 (2004).
Lucas, F. W. S. et al. Electrochemical routes for the valorization of biomass-derived feedstocks: from chemistry to application. ACS Energy Lett. 6, 1205–1270 (2021).
Werpy, T. A., Holladay, J. E. & White, J. F. Top Value Added Chemicals from Biomass: I. Results of Screening for Potential Candidates from Sugars and Synthesis Gas Technical Report (US Department of Energy, 2004); https://doi.org/10.2172/15008859
Zhao, D. Y., Rodriguez-Padron, D., Luque, R. & Len, C. Insights into the selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid using silver oxide. ACS Sustain. Chem. Eng. 8, 8486–8495 (2020).
Deng, Y. L., Handoko, A. D., Du, Y. H., Xi, S. B. & Yeo, B. S. In situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: identification of Cu-III oxides as catalytically active species. ACS Catal. 6, 2473–2481 (2016).
Li, M. et al. Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis. Nat. Catal. 2, 495–503 (2019).
Ursua, A., Gandia, L. M. & Sanchis, P. Hydrogen production from water electrolysis: current status and future trends. Proc. IEEE 100, 410–426 (2012).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Mills, G. & Jonsson, H. Quantum and thermal effects in H2 dissociative adsorption: evaluation of free energy barriers in multidimensional quantum systems. Phys. Rev. Lett. 72, 1124–1127 (1994).
Liu, X. et al. pH effects on the electrochemical reduction of CO2 towards C2 products on stepped copper. Nat. Commun. 10, 32 (2019).
Acknowledgements
This work is supported by the National Key R&D Program of China (grant nos. 2021YFA1500900 (S.W.)), the National Natural Science Foundation of China (grants nos. 21902047 (Y.Z.), 21825201 (S.W.) and U19A2017 (S.W.)) and the Provincial Natural Science Foundation of Hunan (grants nos. 2016TP1009 (S.W.) and 2020JJ5045 (S.W.)).
Author information
Authors and Affiliations
Contributions
S.W. and X.-Z.F. conceived the project. T.W. and L.T. carried out most of the experiments and co-wrote the manuscript. X.Z. and Y.L. performed the theoretical calculations. C.C., W.C., S.D. and Y.Z. performed partial characterization of the materials. B.Z., D.W., P.L., C.X., W.L., Y.W., R.C. and Y.Z. participated in data analysis. X.D. provided some important and constructive suggestions to this work. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Carlos Ponce de León, Lin Zhuang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–30, Discussion, Table 1 and Videos 1 and 2.
Supplementary Video 1
Control experiment of low-potential furfural oxidation using Ag/carbon cloth as the electrode.
Supplementary Video 2
Typical experiment for determining volumes of H2 produced from the anode and cathode of the bipolar hydrogen production system.
Supplementary Data 1
Computational data.
Supplementary Data 2
Statistical source data for Supplementary figures.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
About this article
Cite this article
Wang, T., Tao, L., Zhu, X. et al. Combined anodic and cathodic hydrogen production from aldehyde oxidation and hydrogen evolution reaction. Nat Catal 5, 66–73 (2022). https://doi.org/10.1038/s41929-021-00721-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00721-y
This article is cited by
-
Electrocatalytic hydrogenation of acetonitrile to ethylamine in acid
Nature Communications (2024)
-
Integrating hydrogen utilization in CO2 electrolysis with reduced energy loss
Nature Communications (2024)
-
Pd/NiMoO4/NF electrocatalysts for the efficient and ultra-stable synthesis and electrolyte-assisted extraction of glycolate
Nature Communications (2024)
-
Electrochemical hydrogenation and oxidation of organic species involving water
Nature Reviews Chemistry (2024)
-
Surface engineering of 1-D nanocatalysts for value-added selective electrooxidation of organic chemicals
Nano Research (2024)