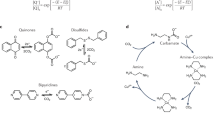
Abstract
Electrochemical CO2 conversion into fuels or chemicals and CO2 capture from point or dilute sources are two important processes to address the gigaton challenges in reducing greenhouse gas emissions. Both CO2 capture and electrochemical CO2 conversion are energy intensive, and synergistic coupling between the two processes can improve the energy efficiency of the system and reduce the cost of the reduced products, via eliminating the CO2 transport and storage or eliminating the capture media regeneration and molecular CO2 release. We consider three different levels to couple electrochemical CO2 reduction with CO2 capture: independent (Type-I), subsequent (Type-II) and fully integrated (Type-III) capture and conversion processes. We focus on Type-II and Type-III configurations and illustrate potential coupling routes of different capture media, which include amine-based solutions and direct carbamate reduction, redox active carriers, aqueous carbonate and bicarbonate solutions, ionic liquids CO2 capture and conversion mediated by covalent organic frameworks.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
20 January 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41929-022-00734-1
References
Jouny, M., Luc, W. & Jiao, F. General techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Xu, H. et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 5, 623–632 (2020).
Cavallaro, N. et al. Second State of the Carbon Cycle Report (US Global Change Research Program, 2018); https://doi.org/10.7930/SOCCR2.2018
McLaren, D. A comparative global assessment of potential negative emissions technologies. Process Saf. Environ. Prot. 90, 489–500 (2012).
Fridahl, M., Hansson, A. & Haikola, S. Towards indicators for a negative emissions climate stabilisation index: problems and prospects. Climate 8, 75 (2020).
National Academies of Sciences, Engineering, and Medicine Negative Emissions Technologies and Reliable Sequestration. Negative Emissions Technologies and Reliable Sequestration (National Academies, 2019); https://doi.org/10.17226/25259
Mac Dowell, N., Fennell, P. S., Shah, N. & Maitland, G. C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 7, 243–249 (2017).
Bettenhausen, C. The life-or-death race to improve carbon capture. Chem. Eng. News 99, 28–35 (2021).
Dutcher, B., Fan, M. & Russell, A. G. Amine-based CO2 capture technology development from the beginning of 2013—a review. ACS Appl. Mater. Interfaces 7, 2137–2148 (2015).
Sharifian, R., Wagterveld, R. M., Digdaya, I. A., Xiang, C. & Vermaas, D. A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781–814 (2021).
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Skydsgaard, N. & Evans, D. World’s largest plant capturing carbon from air starts in Iceland. Reuters (13 September 2021); https://www.reuters.com/business/environment/worlds-largest-plant-capturing-carbon-air-starts-iceland-2021-09-08/
Digdaya, I. A. et al. A direct coupled electrochemical system for capture and conversion of CO2 from oceanwater. Nat. Commun. 11, 4412 (2020).
Eisaman, M. D. et al. CO2 extraction from seawater using bipolar membrane electrodialysis. Energy Environ. Sci. 5, 7346–7352 (2012).
Endrödi, B. et al. Multilayer electrolyzer stack converts carbon dioxide to gas products at high pressure with high efficiency. ACS Energy Lett. 4, 1770–1777 (2019).
Sánchez, O. G. et al. Recent advances in industrial CO2 electroreduction. Curr. Opin. Green. Sustain. Chem. 16, 47–56 (2019).
Stern, M. C., Simeon, F., Herzog, H. & Hatton, T. A. Post-combustion carbon dioxide capture using electrochemically mediated amine regeneration. Energy Environ. Sci. 6, 2505–2517 (2013).
Stern, M. C. & Alan Hatton, T. Bench-scale demonstration of CO2 capture with electrochemically-mediated amine regeneration. RSC Adv. 4, 5906–5914 (2014).
Gurkan, B., Simeon, F. & Hatton, T. A. Quinone reduction in ionic liquids for electrochemical CO2 separation. ACS Sustain. Chem. Eng. 3, 1394–1405 (2015).
Apaydin, D. H., Głowacki, E. D., Portenkirchner, E. & Sariciftci, N. S. Direct electrochemical capture and release of carbon dioxide using an industrial organic pigment: quinacridone. Angew. Chem. Int. Ed. 53, 6819–6822 (2014).
Singh, P. et al. Electrochemical capture and release of carbon dioxide using a disulfide–thiocarbonate redox cycle. J. Am. Chem. Soc. 139, 1033–1036 (2017).
Nagasawa, H., Yamasaki, A., Iizuka, A., Kumagai, K. & Yanagisawa, Y. A new recovery process of carbon dioxide from alkaline carbonate solution via electrodialysis. AIChE J. 55, 3286–3293 (2009).
Watkins, J. D. et al. Redox-mediated separation of carbon dioxide from flue gas. Energy Fuels 29, 7508–7515 (2015).
Renfrew, S. E., Starr, D. E. & Strasser, P. Electrochemical approaches toward CO2 capture and concentration. ACS Catal. 10, 13058–13074 (2020).
Kang, J. S., Kim, S. & Hatton, T. A. Redox-responsive sorbents and mediators for electrochemically based CO2 capture. Curr. Opin. Green Sustain. Chem. 31, 100504 (2021).
Rheinhardt, J. H., Singh, P., Tarakeshwar, P. & Buttry, D. A. Electrochemical capture and release of carbon dioxide. ACS Energy Lett. 2, 454–461 (2017).
Khurram, A., Yan, L., Yin, Y., Zhao, L. & Gallant, B. M. Promoting amine-activated electrochemical CO2 conversion with alkali salts. J. Phys. Chem. C 123, 18222–18231 (2019).
Chen, L. et al. Electrochemical reduction of carbon dioxide in a monoethanolamine capture medium. ChemSusChem 10, 4109–4118 (2017).
Filotás, D., Nagy, T., Nagy, L., Mizsey, P. & Nagy, G. Extended investigation of electrochemical CO2 reduction in ethanolamine solutions by SECM. Electroanalysis 30, 690–697 (2018).
Bhattacharya, M., Sebghati, S., Vercella, Y. M. & Saouma, C. T. Electrochemical reduction of carbamates and carbamic acids: implications for combined carbon capture and electrochemical CO2 recycling. J. Electrochem. Soc. 167, 086507 (2020).
Bhattacharya, M., Sebghati, S., Vanderlinden, R. T. & Saouma, C. T. Toward combined carbon capture and recycling: addition of an amine alters product selectivity from CO to formic acid in manganese catalyzed reduction of CO2. J. Am. Chem. Soc. 142, 17589–17597 (2020).
Margarit, C. G., Asimow, N. G., Costentin, C. & Nocera, D. G. Tertiary amine-assisted electroreduction of carbon dioxide to formate catalyzed by iron tetraphenylporphyrin. ACS Energy Lett. 5, 72–78 (2020).
Abdinejad, M., Mirza, Z., Zhang, X. A. & Kraatz, H. B. Enhanced electrocatalytic activity of primary amines for CO2 reduction using copper electrodes in aqueous solution. ACS Sustain. Chem. Eng. 8, 1715–1720 (2020).
Hossain, M. N., Ahmad, S., da Silva, I. S. & Kraatz, H. B. Electrochemical reduction of CO2 at coinage metal nanodendrites in aqueous ethanolamine. Chem. Eur. J. 27, 1346–1355 (2021).
Li, Y. C. et al. CO2 electroreduction from carbonate electrolyte. ACS Energy Lett. 4, 1427–1431 (2019).
Li, T. et al. Electrolytic conversion of bicarbonate into CO in a flow cell. Joule 3, 1487–1497 (2019).
Rosen, B. A. et al. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 334, 643–644 (2011).
Liu, H. et al. Covalent organic frameworks linked by amine bonding for concerted electrochemical reduction of CO2. Chem 4, 1696–1709 (2018).
Legrand, L., Shu, Q., Tedesco, M., Dykstra, J. E. & Hamelers, H. V. M. Role of ion exchange membranes and capacitive electrodes in membrane capacitive deionization (MCDI) for CO2 capture. J. Colloid Interface Sci. 564, 478–490 (2020).
Liu, Y., Ye, H.-Z., Diederichsen, K. M., Van Voorhis, T. & Hatton, T. A. Electrochemically mediated carbon dioxide separation with quinone chemistry in salt-concentrated aqueous media. Nat. Commun. 11, 2278 (2020).
Lin, Y.-J., Chen, E. & Rochelle, G. T. Pilot plant test of the advanced flash stripper for CO2 capture. Faraday Discuss. 192, 37–58 (2016).
Jens, C. M., Müller, L., Leonhard, K. & Bardow, A. To integrate or not to integrate—techno-economic and life cycle assessment of CO2 capture and conversion to methyl formate using methanol. ACS Sustain. Chem. Eng. 7, 12270–12280 (2019).
Lee, G. et al. Electrochemical upgrade of CO2 from amine capture solution. Nat. Energy 6, 46–53 (2021).
Hori, Y. & Suzuki, S. Electrolytic reduction of bicarbonate ion at a mercury electrode. J. Electrochem. Soc. 130, 2387–2390 (1983).
Aghaie, M., Rezaei, N. & Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: current status and future prospects. Renew. Sustain. Energy Rev. 96, 502–525 (2018).
Park, Y., Lin, K.-Y. A., Park, A.-H. A. & Petit, C. Recent advances in anhydrous solvents for CO2 capture: ionic liquids, switchable solvents, and nanoparticle organic hybrid materials. Front. Energy Res. 3, 42 (2015).
Tanner, E. E. L., Batchelor-McAuley, C. & Compton, R. G. Carbon dioxide reduction in room-temperature ionic liquids: the effect of the choice of electrode material, cation, and anion. J. Phys. Chem. C 120, 26442–26447 (2016).
Feaster, J. T. et al. Understanding the influence of [EMIM]Cl on the suppression of the hydrogen evolution reaction on transition metal electrodes. Langmuir 33, 9464–9471 (2017).
Rosen, B. A. et al. In situ spectroscopic examination of a low overpotential pathway for carbon dioxide conversion to carbon monoxide. J. Phys. Chem. C. 116, 15307–15312 (2012).
Yang, Z. Z., Zhao, Y. N. & He, L. N. CO2 chemistry: task-specific ionic liquids for CO2 capture/activation and subsequent conversion. RSC Adv. 1, 545–567 (2011).
Bates, E. D., Mayton, R. D., Ntai, I. & Davis, J. H. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 124, 926–927 (2002).
Luo, X. et al. Significant improvements in CO2 capture by pyridine-containing anion-functionalized ionic liquids through multiple-site cooperative interactions. Angew. Chem. Int. Ed. 53, 7053–7057 (2014).
Medina-Ramos, J. et al. Structural dynamics and evolution of bismuth electrodes during electrochemical reduction of CO2 in imidazolium-based ionic liquid solutions. ACS Catal. 7, 7285–7295 (2017).
Zeng, Y., Zou, R. & Zhao, Y. Covalent organic frameworks for CO2 capture. Adv. Mater. 28, 2855–2873 (2016).
Johnson, E. M., Haiges, R. & Marinescu, S. C. Covalent–organic frameworks composed of rhenium bipyridine and metal porphyrins: designing heterobimetallic frameworks with two distinct metal sites. ACS Appl. Mater. Interfaces 10, 37919–37927 (2018).
Su, P., Iwase, K., Harada, T., Kamiya, K. & Nakanishi, S. Covalent triazine framework modified with coordinatively-unsaturated Co or Ni atoms for CO2 electrochemical reduction. Chem. Sci. 9, 3941–3947 (2018).
Wu, Q. et al. Integration of strong electron transporter tetrathiafulvalene into metalloporphyrin-based covalent organic framework for highly efficient electroreduction of CO2. ACS Appl. Mater. Interfaces 5, 1005–1012 (2020).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Lin, C. Y., Zhang, D., Zhao, Z. & Xia, Z. Covalent organic framework electrocatalysts for clean energy conversion. Adv. Mater. 30, 170364 (2018).
Wang, Y., Chen, J., Wang, G., Li, Y. & Wen, Z. Perfluorinated covalent triazine framework derived hybrids for the highly selective electroconversion of carbon dioxide into methane. Angew. Chem. Int. Ed. 57, 13120–13124 (2018).
Uribe-Romo, F. J. et al. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 131, 4570–4571 (2009).
Acknowledgements
This material is based on work performed by the Liquid Sunlight Alliance, which is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, and Fuels from Sunlight Hub under award no. DE-SC0021266. We also acknowledge the support from SoCalGas on the analysis of CO2 capture processes under award no. 5660060287. This research received funding from the Netherlands Organization for Scientific Research (NWO) under project no. 733.000.008 in the framework of the Solar to Products programme co-funded by Shell Global Solutions International B.V., and from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 852115).
Author information
Authors and Affiliations
Contributions
C.X., H.A.A. and D.A.V. conceptualized and organized different levels of coupling between electrochemical CO2 conversion with CO2 capture in the manuscript. I.S. and A.G. contributed to writing and editing of the various approaches for coupling CO2 capture with CO2 conversion. I.A.D. and X.L. contributed to preparing the figures and references, as well as editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Caroline Saouma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sullivan, I., Goryachev, A., Digdaya, I.A. et al. Coupling electrochemical CO2 conversion with CO2 capture. Nat Catal 4, 952–958 (2021). https://doi.org/10.1038/s41929-021-00699-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00699-7
This article is cited by
-
Integrated energy storage and CO2 conversion using an aqueous battery with tamed asymmetric reactions
Nature Communications (2024)
-
Complementary probes for the electrochemical interface
Nature Reviews Chemistry (2024)
-
Zeolite-confined Fe-site Catalysts for the Hydrogenation of CO2 to Produce High-value Chemicals
Chemical Research in Chinese Universities (2024)
-
C60 as a metal-free catalyst for lithium-oxygen batteries
Nano Research (2024)
-
The role of ionic blockades in controlling the efficiency of energy recovery in forward bias bipolar membranes
Nature Energy (2023)