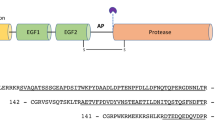
Abstract
Zymogen (prothrombin) activation is central to the process of haemostasis (blood clotting) in the body, preventing serious blood loss and death from haemorrhagic shock. Zeolites comprise a family of crystalline microporous aluminosilicates that show increasing promise for use in massive bleeding control. However, the mechanism of zeolite-initiated haemostasis has remained unclear. Here, we investigate zeolite-initiated thrombin activation at the molecular level, and show that a prothrombinase complex can assemble on the inorganic surface of calcium-ion-exchanged zeolites (Ca-zeolites). Compared to natural platelet-based physiological processes, prothrombin-to-thrombin conversion on the surface of Ca-zeolite displays a striking thrombin activation pattern, with an exceptionally high plateau thrombin activity and at least 12-fold enhanced endogenous thrombin. The results provide a mechanistic understanding of how the zeolite surface functionally contributes to thrombin activation, paving the way towards the design of improved agents for bleeding management.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information or from the corresponding authors upon reasonable request. The MS proteomics data have been deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD024170. Source data are provided with this paper.
References
Neurath, H. Evolution of proteolytic enzymes. Science 224, 350–357 (1984).
Neurath, H. & Walsh, K. A. Role of proteolytic enzymes in biological regulation (a review). Proc. Natl Acad. Sci. USA 73, 3825–3832 (1976).
Wolberg, A. S. & Campbell, R. A. Thrombin generation, fibrin clot formation and hemostasis. Transfus. Apher. Sci. 38, 15–23 (2008).
Monroe, D. M., Hoffman, M. & Roberts, H. R. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 22, 1381–1389 (2002).
Wolberg, A. S. Thrombin generation and fibrin clot structure. Blood Rev. 21, 131–142 (2007).
Monroe, D. M. & Hoffman, M. What does it take to make the perfect clot? Arterioscler. Thromb. Vasc. Biol. 26, 41–48 (2006).
Hickman, D. A., Pawlowski, C. L., Sekhon, U. D., Marks, J. & Gupta, A. S. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv. Mater. 30, 1700859 (2018).
Alam, H. B. et al. Application of a zeolite hemostatic agent achieves 100% survival in a lethal model of complex groin injury in swine. J. Trauma Acute Care Surg. 56, 974–983 (2004).
Kheirabadi, B. S., Scherer, M. R., Estep, J. S., Dubick, M. A. & Holcomb, J. B. Determination of efficacy of new hemostatic dressings in a model of extremity arterial hemorrhage in swine. J. Trauma Acute Care Surg. 67, 450–460 (2009).
Gruen, R. L. et al. Haemorrhage control in severely injured patients. Lancet 380, 1099–1108 (2012).
Achneck, H. E. et al. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann. Surg. 251, 217–228 (2010).
Gordy, S. D., Rhee, P. & Schreiber, M. A. Military applications of novel hemostatic devices. Expert Rev. Med. Devices 8, 41–47 (2011).
Pourshahrestani, S., Zeimaran, E., Djordjevic, I., Kadri, N. A. & Towler, M. R. Inorganic hemostats: the state-of-the-art and recent advances. Mater. Sci. Eng. C 58, 1255–1268 (2016).
Yu, L. et al. A tightly-bonded and flexible mesoporous zeolite–cotton hybrid hemostat. Nat. Commun. 10, 1932 (2019).
Granville-Chapman, J., Jacobs, N. & Midwinter, M. Pre-hospital haemostatic dressings: a systematic review. Injury 42, 447–459 (2011).
Li, Y. et al. In situ generated thrombin in the protein corona of zeolites: relevance of the functional proteins to its biological impact. Nano Res. 7, 1457–1465 (2014).
Tenzer, S. et al. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysis. ACS Nano 5, 7155–7167 (2011).
Walkey, C. D. & Chan, W. C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 41, 2780–2799 (2012).
Cedervall, T. et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl Acad. Sci. USA 104, 2050–2055 (2007).
Monopoli, M. P., Åberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 7, 779–786 (2012).
Caracciolo, G. et al. Stealth effect of biomolecular corona on nanoparticle uptake by immune cells. Langmuir 31, 10764–10773 (2015).
Dobrovolskaia, M. A. & McNeil, S. E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2, 469–478 (2007).
Kelly, P. M. et al. Mapping protein binding sites on the biomolecular corona of nanoparticles. Nat. Nanotechnol. 10, 472–479 (2015).
Dawson, K. A. & Yan, Y. Current understanding of biological identity at the nanoscale and future prospects. Nat. Nanotechnol. 16, 229–242 (2021).
Jain, P. et al. In-vitro in-vivo correlation (IVIVC) in nanomedicine: is protein corona the missing link? Biotechnol. Adv. 35, 889–904 (2017).
Monopoli, M. P. et al. Physical–chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 133, 2525–2534 (2011).
Winzen, S. et al. Complementary analysis of the hard and soft protein corona: sample preparation critically effects corona composition. Nanoscale 7, 2992–3001 (2015).
Spahn, D. R. et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit. Care 17, R76 (2013).
Chapin, J. C. & Hajjar, K. A. Fibrinolysis and the control of blood coagulation. Blood Rev. 29, 17–24 (2015).
Foley, J. H. Plasmin(ogen) at the nexus of fibrinolysis, inflammation and complement. Semin. Thromb. Hemost. 43, 135–142 (2017).
Laurent, S. et al. Corona protein composition and cytotoxicity evaluation of ultra-small zeolites synthesized from template free precursor suspensions. Toxicol. Res. 2, 270–279 (2013).
Rahimi, M. et al. Zeolite nanoparticles for selective sorption of plasma proteins. Sci. Rep. 5, 17259 (2015).
Krishnaswamy, S. The transition of prothrombin to thrombin. J. Thromb. Haemost. 11, 265–276 (2013).
Ivanciu, L., Krishnaswamy, S. & Camire, R. M. New insights into the spatiotemporal localization of prothrombinase in vivo. Blood 124, 1705–1714 (2014).
Crawley, J., Zanardelli, S., Chion, C. & Lane, D. The central role of thrombin in hemostasis. J. Thromb. Haemost. 5, 95–101 (2007).
Allen, G. A. et al. Impact of procoagulant concentration on rate, peak and total thrombin generation in a model system. J. Thromb. Haemost. 2, 402–413 (2004).
Lane, D. A., Philippou, H. & Huntington, J. A. Directing thrombin. Blood 106, 2605–2612 (2005).
Tomaiuolo, M., Brass, L. F. & Stalker, T. J. Regulation of platelet activation and coagulation and its role in vascular injury and arterial thrombosis. Interv. Cardiol. Clin. 6, 1–12 (2017).
Wilf, J., Gladner, J. A. & Minton, A. P. Acceleration of fibrin gel formation by unrelated proteins. Thromb. Res. 37, 681–688 (1985).
Torbet, J. Fibrin assembly in human plasma and fibrinogen/albumin mixtures. Biochemistry 25, 5309–5314 (1986).
Daniel, R. M., Dunn, R. V., Finney, J. L. & Smith, J. C. The role of dynamics in enzyme activity. Annu. Rev. Biophys. Biomol. Struct. 32, 69–92 (2003).
Zhou, Y. et al. Probing the lysine proximal microenvironments within membrane protein complexes by active dimethyl labeling and mass spectrometry. Anal. Chem. 88, 12060–12065 (2016).
Huntington, J. Molecular recognition mechanisms of thrombin. J. Thromb. Haemost. 3, 1861–1872 (2005).
Ricci, C. G., Pinto, A. F. M., Berger, M. & Termignoni, C. A thrombin inhibitor from the gut of Boophilus microplus ticks. Exp. Appl. Acarol. 42, 291–300 (2007).
Hursey, F. X. & Dechene, F. J. Method of treating wounds. US patent 4,822,349 (1989).
Ostomel, T. A., Stoimenov, P. K., Holden, P. A., Alam, H. B. & Stucky, G. D. Host–guest composites for induced hemostasis and therapeutic healing in traumatic injuries. J. Thromb. Thrombolysis 22, 55–67 (2006).
Sloan, I. G. & Firkin, B. G. Effects of EACA on thrombin generation as measured by the chromagen S2238. Thromb. Res. 44, 761–769 (1986).
Acknowledgements
J.F. acknowledges support from the National Natural Science Foundation of China (91545113, 91845203 and 92045301), China Postdoctoral Science Foundation (2017M610363 and 2018T110584), Shell Global Solutions International BV (PT71423 and PT74557), Fok Ying Tong Education Foundation (131015) and the Science & Technology Program of Ningbo (2017C50014). K.A.D. acknowledges that this publication has emanated from research supported in part by grants from Science Foundation Ireland (17/NSFC/4898 and 17/ERCD/4962) and also from funding under Guangdong Provincial Education Department Key Laboratory of Nano-Immunoregulation Tumor Microenvironment (2019KSYS008). V.C. acknowledges support from the Irish Research Council (GOIPD/2016/128). L.B. acknowledges financial support from the EU H2020 Nanofacturing project (grant agreement no. 646364). V.P. acknowledges financial support from CALIN. We thank Q. Gao for helping with proteomic analysis and protein structure analysis.
Author information
Authors and Affiliations
Contributions
J.F. and K.A.D. conceived the project. J.F., K.A.D., X.S. and H.C. designed the experiments and wrote the manuscript. X.S. and H.C. carried out the experiments and data analysis. K.L., V.C., L.B. and V.P. helped with the protein corona MS analysis. M.H. and F.W. helped with the protein conformation analysis. L.X. and L.Y. helped with revision of the manuscript. All authors discussed the results and contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Russell Morris, Ivan D Tarandovskiy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Methods, Tables 1–7 and Additional Supplementary Fig. 1.
Supplementary Data 1
Statistical source data for Supplementary Figs. 2, 3, 6, 8, 9 and 11.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shang, X., Chen, H., Castagnola, V. et al. Unusual zymogen activation patterns in the protein corona of Ca-zeolites. Nat Catal 4, 607–614 (2021). https://doi.org/10.1038/s41929-021-00654-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00654-6
This article is cited by
-
Application and progress of inorganic composites in haemostasis: a review
Journal of Materials Science (2024)
-
Structural characterization of protein-material interfacial interactions using lysine reactivity profiling-mass spectrometry
Nature Protocols (2023)
-
Measurements of heterogeneity in proteomics analysis of the nanoparticle protein corona across core facilities
Nature Communications (2022)
-
The interfacial interactions of nanomaterials with human serum albumin
Analytical and Bioanalytical Chemistry (2022)