Abstract
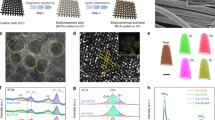
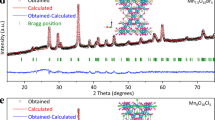
Achieving stable, low-cost electrocatalysts represents a daunting challenge towards practical water oxidation reactions. Here, we report that a degraded electrocatalyst can be revivified under catalytic operating conditions by manipulating reversible phase segregation. Under the oxygen evolution reaction conditions, Fe segregation develops in the Ni–Fe hydroxide host lattice, with the formation of FeOOH, resulting in an interface between the FeOOH and the host lattice. A dynamic metal dissolution–redeposition process accelerates the Fe segregation and formation of the FeOOH secondary phase, resulting in catalyst deactivation. Operando synchrotron spectroscopic and microscopic analyses suggest that the phase segregation is reversible between the water oxidation potential and the catalyst reduction potential. Therefore, we have developed an intermittent reduction methodology to revivify the catalytic activity under the operating conditions, enhancing catalyst durability. The present study highlights that tailoring phase segregation at the catalyst/electrolyte interface constitutes an important strategy for revivifying and stabilizing catalytic activity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Irvine, J. T. S. et al. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 1, 15014–15016 (2016).
Cui, C., Gan, L., Heggen, M., Rudi, S. & Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 12, 765–771 (2013).
Lin, F. et al. Metal segregation in hierarchically structured cathode materials for high-energy lithium batteries. Nat. Energy 1, 15004–15011 (2016).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014 (2016).
Gao, Z. W. et al. Engineering NiO/NiFe LDH intersection to bypass scaling relationship for oxygen evolution reaction via dynamic tridimensional adsorption of intermediates. Adv. Mater. 31, e1804769 (2019).
Huang, Z.-F. et al. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 4, 329–338 (2019).
Li, S. et al. Ir–O–V catalytic group in Ir-doped NiV(OH)2 for overall water splitting. ACS Energy Lett. 4, 1823–1829 (2019).
Wu, T. et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2, 763–772 (2019).
Lopes, P. P. et al. Relationships between atomic level surface structure and stability/activity of platinum surface atoms in aqueous environments. ACS Catal. 6, 2536–2544 (2016).
Bergmann, A. et al. Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat. Commun. 6, 8625 (2015).
Lee, W., Han, J. W., Chen, Y., Cai, Z. & Yildiz, B. Cation size mismatch and charge interactions drive dopant segregation at the surfaces of manganite perovskites. J. Am. Chem. Soc. 135, 7909–7925 (2013).
Chen, R. et al. Layered structure causes bulk NiFe layered double hydroxide unstable in alkaline oxygen evolution reaction. Adv. Mater. 31, e1903909 (2019).
Polo-Garzon, F., Bao, Z., Zhang, X., Huang, W. & Wu, Z. Surface reconstructions of metal oxides and the consequences on catalytic chemistry. ACS Catal. 9, 5692–5707 (2019).
Koo, B. et al. Sr segregation in perovskite oxides: why it happens and how it exists. Joule 2, 1476–1499 (2018).
Zeng, Z., Chang, K.-C., Kubal, J., Markovic, N. M. & Greeley, J. Stabilization of ultrathin (hydroxy)oxide films on transition metal substrates for electrochemical energy conversion. Nat. Energy 2, 17070–17079 (2017).
Maljusch, A., Conradi, O., Hoch, S., Blug, M. & Schuhmann, W. Advanced evaluation of the long-term stability of oxygen evolution electrocatalysts. Anal. Chem. 88, 7597–7602 (2016).
Kuai, C. et al. Fully oxidized Ni–Fe layered double hydroxide with 100% exposed active sites for catalyzing oxygen evolution reaction. ACS Catal. 9, 6027–6032 (2019).
Chen, G. et al. An amorphous nickel–iron-based electrocatalyst with unusual local structures for ultrafast oxygen evolution reaction. Adv. Mater. 31, e1900883 (2019).
Duan, Y. et al. Scaled-up synthesis of amorphous NiFeMo oxides and their rapid surface reconstruction for superior oxygen evolution catalysis. Angew. Chem. 58, 15772–15777 (2019).
Corrigan, A. D. The catalysis of the oxygen evolution reaction by iron impurities in thin film nickel oxide electrodes. J. Electrochem. Soc. 134, 377–384 (1987).
Trotochaud, L., Young, S. L., Ranney, J. K. & Boettcher, S. W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 136, 6744–6753 (2014).
Friebel, D. et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Gorlin, M. et al. Oxygen evolution reaction dynamics, faradaic charge efficiency, and the active metal redox states of Ni–Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc. 138, 5603–5614 (2016).
Li, N. et al. Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films. Proc. Natl Acad. Sci. USA 114, 1486–1491 (2017).
Bergmann, A. et al. Unified structural motifs of the catalytically active state of Co(oxyhydr)oxides during the electrochemical oxygen evolution reaction. Nat. Catal. 1, 711–719 (2018).
Qiu, Z., Tai, C.-W., Niklasson, G. A. & Edvinsson, T. Direct observation of active catalyst surface phases and the effect of dynamic self-optimization in NiFe-layered double hydroxides for alkaline water splitting. Energy Environ. Sci. 12, 572–581 (2019).
Huber, A. K. et al. In situ study of electrochemical activation and surface segregation of the SOFC electrode material La0.75Sr0.25Cr0.5Mn0.5O(3+/–𝛿). Phys. Chem. Chem. Phys. 14, 751–758 (2012).
Graves, C., Ebbesen, S. D., Jensen, S. H., Simonsen, S. B. & Mogensen, M. B. Eliminating degradation in solid oxide electrochemical cells by reversible operation. Nat. Mater. 14, 239–244 (2015).
Danilovic, N. et al. Using surface segregation to design stable Ru-Ir oxides for the oxygen evolution reaction in acidic environments. Angew. Chem. 53, 14016–14021 (2014).
Rahman, M. M. et al. Empowering multicomponent cathode materials for sodium ion batteries by exploring three-dimensional compositional heterogeneities. Energy Environ. Sci. 11, 2496–2508 (2018).
Lin, R. et al. Anomalous metal segregation in lithium-rich material provides design rules for stable cathode in lithium-ion battery. Nat. Commun. 10, 1650 (2019).
Hu, S. M. Formation of stacking faults and enhanced diffusion in the oxidation of silicon. J. Appl. Phys. 45, 1567–1573 (1974).
Zhang, S. B. & Northrup, J. E. Chemical potential dependence of defect formation energies in GaAs: application to Ga self-diffusion. Phys. Rev. Lett. 67, 2339–2342 (1991).
Litton, D. A. & Garofalini, S. H. Vitreous silica bulk and surface self-diffusion analysis by molecular dynamics. J. Non-Cryst. Solids 217, 250–263 (1997).
Deng, J. et al. Morphology dynamics of single-layered Ni(OH)2/NiOOH nanosheets and subsequent Fe incorporation studied by in situ electrochemical atomic force microscopy. Nano Lett. 17, 6922–6926 (2017).
Chang, S. H. et al. Activity–stability relationship in the surface electrochemistry of the oxygen evolution reaction. Faraday Discuss. 176, 125–133 (2014).
Jia, Q. et al. Roles of Mo surface dopants in enhancing the ORR performance of octahedral PtNi nanoparticles. Nano Lett. 18, 798–804 (2018).
Wang, W., Luo, J. & Chen, S. Carbon oxidation reactions could misguide the evaluation of carbon black-based oxygen-evolution electrocatalysts. Chem. Commun. 53, 11556–11559 (2017).
Westre, T. E. et al. A multiplet analysis of Fe K-edge 1s → 3d pre-edge features of iron complexes. J. Am. Chem. Soc. 119, 6297–6314 (1997).
Funke, H., Scheinost, A. C. & Chukalina, M. Wavelet analysis of extended X-ray absorption fine structure data. Phys. Rev. B 71, 094110 (2005).
Funke, H., Chukalina, M. & Scheinost, A. C. A new FEFF-based wavelet for EXAFS data analysis. J. Synchrotron Radiat. 14, 426–432 (2007).
Stevens, M. B. et al. Measurement techniques for the study of thin film heterogeneous water oxidation electrocatalysts. Chem. Mater. 29, 120–140 (2016).
Smith, R. D. L. et al. Geometric distortions in nickel (oxy)hydroxide electrocatalysts by redox inactive iron ions. Energy Environ. Sci. 11, 2476–2485 (2018).
Zou, S. et al. Fe (oxy)hydroxide oxygen evolution reaction electrocatalysis: intrinsic activity and the roles of electrical conductivity, substrate, and dissolution. Chem. Mater. 27, 8011–8020 (2015).
Stevens, M. B., Trang, C. D. M., Enman, L. J., Deng, J. & Boettcher, S. W. Reactive Fe-sites in Ni/Fe (oxy)hydroxide are responsible for exceptional oxygen electrocatalysis activity. J. Am. Chem. Soc. 139, 11361–11364 (2017).
Corrigan, D. A. Electrochemical and spectroscopic evidence on the participation of quadrivalent nickel in the nickel hydroxide redox reaction. J. Electrochem. Soc. 136, 613–619 (1989).
Shin, H., Xiao, H. & Goddard, W. A. III In silico discovery of new dopants for Fe-doped Ni oxyhydroxide (Ni1–xFexOOH) catalysts for oxygen evolution reaction. J. Am. Chem. Soc. 140, 6745–6748 (2018).
Dionigi, F. et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 11, 2522 (2020).
Bediako, D. K. et al. Structure–activity correlations in a nickel–borate oxygen evolution catalyst. J. Am. Chem. Soc. 134, 6801–6809 (2012).
Gorlin, M. et al. Tracking catalyst redox states and reaction dynamics in Ni–Fe oxyhydroxide oxygen evolution reaction electrocatalysts: the role of catalyst support and electrolyte pH. J. Am. Chem. Soc. 139, 2070–2082 (2017).
de Jonge, M. D. & Vogt, S. Hard X-ray fluorescence tomography—an emerging tool for structural visualization. Curr. Opin. Struct. Biol. 20, 606–614 (2010).
Vogt, S. MAPS: a set of sortware tools for analysis and visualization of 3D X-ray fluorescence data sets. J. Phys. IV Fr. 104, 635–637 (2003).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Acknowledgements
This work was supported by the Department of Chemistry Startup Funds and the Institute for Critical Technology and Applied Science at Virginia Tech. The work at Tianjin university was supported by the Natural Science Foundation of China (grant nos. 51871160, 51671141 and 51471115). This research used the resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, was supported by the US DOE, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515. The authors thank S. Li and Y. Liu of SLAC for assisting the development of the synchrotron operando cells, and W. Liu for assisting the XFM measurements at APS 34-ID-E.
Author information
Authors and Affiliations
Contributions
F.L. and X.W.D. conceived the project. F.L. led the project. C.G.K., F.L. and X.W.D. designed the experiments. C.G.K. synthesized the materials and performed characterization and electrochemical measurements. C.G.K., Z.X., Z.Y. and Y.Z. performed the synchrotron XAS experiments with the assistance of C.-J.S. and D.S. C.G.K., Z.X. and L.L. performed the synchrotron XFM experiments. A.H. assisted with the electrochemical and ICP-MS measurements. C.X. conducted the DFT calculations under the supervision of C.K.D. S.Q. participated in the scientific discussion. C.G.K., F.L. and X.W.D. prepared the figures and wrote the manuscript with the assistance of all the other co-authors. All of the co-authors participated in the scientific discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28, Tables 1–3 and Notes 1 and 2
Supplementary Data 1
Atomic coordinates of the calculated Ni0.75Fe0.25(OH)2 structure where Fe ions are segregated at the edge.
Supplementary Data 2
Atomic coordinates of the calculated Ni0.75Fe0.25(OH)2 structure where Fe ions are uniformly distributed.
Supplementary Data 3
Atomic coordinates of the calculated K1/3(Ni3/4Fe1/4)O2 structure where Fe ions are segregated at the edge.
Supplementary Data 4
Atomic coordinates of the calculated K1/3(Ni3/4Fe1/4)O2 structure where Fe ions are segregated in bulk.
Supplementary Data 5
Atomic coordinates of the calculated K1/3(Ni3/4Fe1/4)O2 structure where Fe ions are uniformly distributed.
Supplementary Data 6
Atomic coordinates of the calculated K1/3(Ni5/6Fe1/6)O2 structure where Fe ions are segregated at the edge.
Supplementary Data 7
Atomic coordinates of the calculated K1/3(Ni5/6Fe1/6)O2 structure where Fe ions are segregated in bulk.
Supplementary Data 8
Atomic coordinates of the calculated K1/3(Ni5/6Fe1/6)O2 structure where Fe ions are uniformly distributed.
Rights and permissions
About this article
Cite this article
Kuai, C., Xu, Z., Xi, C. et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts. Nat Catal 3, 743–753 (2020). https://doi.org/10.1038/s41929-020-0496-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0496-z
This article is cited by
-
A restricted dynamic surface self-reconstruction toward high-performance of direct seawater oxidation
Nature Communications (2024)
-
Bioinspired trimesic acid anchored electrocatalysts with unique static and dynamic compatibility for enhanced water oxidation
Nature Communications (2023)
-
Lamella-heterostructured nanoporous bimetallic iron-cobalt alloy/oxyhydroxide and cerium oxynitride electrodes as stable catalysts for oxygen evolution
Nature Communications (2023)
-
Operando characterization and regulation of metal dissolution and redeposition dynamics near battery electrode surface
Nature Nanotechnology (2023)
-
Activating lattice oxygen in high-entropy LDH for robust and durable water oxidation
Nature Communications (2023)