Abstract
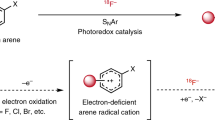
Nucleophilic aromatic substitution (SNAr) is routinely used to install 19F– and 18F– in aromatic molecules, but is typically limited to electron-deficient arenes due to kinetic barriers associated with C–F bond formation. Here we demonstrate that a polarity-reversed photoredox-catalysed arene deoxyfluorination that operates via cation-radical-accelerated SNAr enables the fluorination of electron-rich arenes with 19F– and 18F– under mild conditions, and thus complements the traditional arene polarity requirements necessary for SNAr-based fluorination. The utility of our radiofluorination strategy is highlighted by short reaction times, compatibility with multiple nucleofuges and high radiofluorination yields, especially that of an important cancer positron emission tomography agent [18F]5-fluorouracil. Taken together, our fluorination approach enables the development of fluorinated and radiofluorinated compounds that can be difficult to access by classical SNAr strategies, with the potential for use in the synthesis and discovery of positron emission tomography radiopharmaceuticals.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information or from the authors upon reasonable request.
References
Wang, J. et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 114, 2432–2506 (2014).
Zhou, Y. et al. Next generation of fluorine-containing pharmaceuticals, compounds currently in Phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 116, 422–518 (2016).
Fujiwara, T. & O’Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 167, 16–29 (2014).
Preshlock, S., Tredwell, M. & Gouverneur, V. 18F-labeling of arenes and heteroarenes for applications in positron emission tomography. Chem. Rev. 116, 719–766 (2016).
Coenen, H. H. et al. Fluorine-18 radiopharmaceuticals beyond [18F]FDG for use in oncology and neurosciences. Nucl. Med. Biol. 37, 727–740 (2010).
Terrier, F. Modern Nucleophilic Aromatic Substitution (Wiley, 2013).
Deng, X. et al. Chemistry for positron emission tomography: recent advances in 11C-, 18F-, 13N-, and 15O-labeling reactions. Angew. Chem. Int. Ed. 58, 2580–2605 (2019).
Kwan, E. E., Zeng, Y., Besser, H. A. & Jacobsen, E. N. Concerted nucleophilic aromatic substitutions. Nat. Chem. 10, 917–923 (2018).
Neumann, C. N., Hooker, J. M. & Ritter, T. Concerted nucleophilic aromatic substitution with 19F− and 18F−. Nature 534, 369–373 (2016).
Watson, D. A. et al. Formation of ArF from LPdAr(F): catalytic conversion of aryl triflates to aryl fluorides. Science 325, 1661–1664 (2009).
Sather, A. C. et al. A fluorinated ligand enables room-temperature and regioselective Pd-catalyzed fluorination of aryl triflates and bromides. J. Am. Chem. Soc. 137, 13433–13438 (2015).
Ye, Y., Schimler, S. D., Hanley, P. S. & Sanford, M. S. Cu(OTf)2-mediated fluorination of aryltrifluoroborates with potassium fluoride. J. Am. Chem. Soc. 135, 16292–16295 (2013).
Furuya, T., Kaiser, H. M. & Ritter, T. Palladium-mediated fluorination of arylboronic acids. Angew. Chem. Int. Ed. 47, 5993–5996 (2008).
Ichiishi, N. et al. Copper-catalyzed [18F]fluorination of (mesityl)(aryl)iodonium salts. Org. Lett. 16, 3224–3227 (2014).
Campbell, M. G. & Ritter, T. Modern carbon–fluorine bond forming reactions for aryl fluoride synthesis. Chem. Rev. 115, 612–633 (2015).
Cardinale, J. et al. Carrier-effect on palladium-catalyzed, nucleophilic 18F-fluorination of aryl triflates. J. Label. Compd Radiopharm. 55, 450–453 (2012).
Tang, P., Wang, W. & Ritter, T. Deoxyfluorination of phenols. J. Am. Chem. Soc. 133, 11482–11484 (2011).
Schimler, S. D. et al. Nucleophilic deoxyfluorination of phenols via aryl fluorosulfonate intermediates. J. Am. Chem. Soc. 139, 1452–1455 (2017).
Schimler, S. D., Froese, R. D. J., Bland, D. C. & Sanford, M. S. Reactions of arylsulfonate electrophiles with NMe4F: mechanistic insight, reactivity, and scope. J. Org. Chem. 83, 11178–11190 (2018).
Beyzavi, M. H. et al. 18F-deoxyfluorination of phenols via Ru π-complexes. ACS Cent. Sci. 3, 944–948 (2017).
Tay, N. E. S. & Nicewicz, D. A. Cation radical accelerated nucleophilic aromatic substitution via organic photoredox catalysis. J. Am. Chem. Soc. 139, 16100–16104 (2017).
Holmberg-Douglas, N. & Nicewicz, D. A. Arene cyanation via cation-radical accelerated-nucleophilic aromatic substitution. Org. Lett. 21, 7114–7118 (2019).
Chen, W. et al. Direct arene C–H fluorination with 18F− via organic photoredox catalysis. Science 364, 1170–1174 (2019).
Zweig, A., Hodgson, W. G. & Jura, W. H. The oxidation of methoxybenzenes. J. Am. Chem. Soc. 86, 4124–4129 (1964).
Um, I.-H., Kim, M.-Y. & Dust, J. M. Medium effect (water versus MeCN) on reactivity and reaction pathways for the SNAr reaction of 1-aryloxy-2,4-dinitrobenzenes with cyclic secondary amines. Can. J. Chem. 95, 1273–1279 (2017).
Schmittel, M. & Burghart, A. Understanding reactivity patterns of radical cations. Angew. Chem. Int. Ed. Engl. 36, 2550–2589 (1997).
Nolte, C., Ammer, J. & Mayr, H. Nucleofugality and nucleophilicity of fluoride in protic solvents. J. Org. Chem. 77, 3325–3335 (2012).
Kim, D. W. et al. A new class of SN2 reactions catalyzed by protic solvents: facile fluorination for isotopic labeling of diagnostic molecules. J. Am. Chem. Soc. 128, 16394–16397 (2006).
Kim, D. W. et al. Facile nucleophilic fluorination reactions using tert-alcohols as a reaction medium: Significantly enhanced reactivity of alkali metal fluorides and improved selectivity. J. Org. Chem. 73, 957–962 (2008).
Kim, D. W., Jeong, H.-J., Lim, S. T. & Sohn, M.-H. Tetrabutylammonium tetra(tert-butyl alcohol)-coordinated fluoride as a facile fluoride source. Angew. Chem. Int. Ed. 47, 8404–8406 (2008).
Sharma, R. K. & Fry, J. L. Instability of anhydrous tetra-n-alkylammonium fluorides. J. Org. Chem. 48, 2112–2114 (1983).
Sun, H. & DiMagno, S. G. Anhydrous tetrabutylammonium fluoride. J. Am. Chem. Soc. 127, 2050–2051 (2005).
Lee, J.-W. et al. Hydrogen-bond promoted nucleophilic fluorination: concept, mechanism and applications in positron emission tomography. Chem. Soc. Rev. 45, 4638–4650 (2016).
Hoover, A. J. et al. A transmetalation reaction enables the synthesis of [18F]5-fluorouracil from [18F]fluoride for human PET imaging. Organometallics 35, 1008–1014 (2016).
Webber, E. M., Kauffman, T. L., O’Connor, E. & Goddard, K. A. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer 15, 156 (2015).
Lemaire, C. F. et al. Fast production of highly reactive no-carrier-added [18F]fluoride for the labeling of radiopharmaceuticals. Angew. Chem. Int. Ed. 49, 3161–3164 (2010).
Roth, H. G., Romero, N. A. & Nicewicz, D. A. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett 27, 714–723 (2016).
Fowler, J. S., Finn, R. D., Lambrecht, R. M. & Wolf, A. P. The synthesis of 18F-5-fluorouracil. VII. J. Nucl. Med. 14, 63–64 (1973).
Saleem, A. et al. Modulation of fluorouracil tissue pharmacokinetics by eniluracil: in-vivo imaging of drug action. Lancet 355, 2125–2131 (2000).
Rotstein, B. H., Stephenson, N. A., Vasdev, N. & Liang, S. H. Spirocyclic hypervalent iodine(iii)-mediated radiofluorination of non-activated and hindered aromatics. Nat. Commun. 5, 4365 (2014).
Sulkes, A., Benner, S. E. & Canetta, R. M. Uracil-ftorafur: an oral fluoropyrimidine active in colorectal cancer. J. Clin. Oncol. 16, 3461–3475 (1998).
Álvarez, P. et al. 5-Fluorouracil derivatives: a patent review. Expert Opin. Ther. Pat. 22, 107–123 (2012).
Li, M., Liang, Z., Sun, X., Gong, T. & Zhang, Z. A polymeric prodrug of 5-fluorouracil-1-acetic acid using a multi-hydroxyl polyethylene glycol derivative as the drug carrier. PLoS ONE 9, e112888 (2014).
Sergeev, M. et al. Performing radiosynthesis in microvolumes to maximize molar activity of tracers for positron emission tomography. Commun. Chem. 1, 1–10 (2018).
Sun, Y. et al. The preliminary study of 16α-[18F]fluoroestradiol PET/CT in assisting the individualized treatment decisions of breast cancer patients. PLoS ONE 10, e0116341 (2015).
Liao, G. J., Clark, A. S., Schubert, E. K. & Mankoff, D. A. 18F-fluoroestradiol PET: current status and potential future clinical applications. J. Nucl. Med. 57, 1269–1275 (2016).
Acknowledgements
Financial support was provided in part by the National Institutes of Health (NIBIB) 5R01EB014354 (Z.L.), R01EB029451 (Z.L. and D.A.N.), by the UNC Department of Radiology, Biomedical Research Imaging Center and by the UNC Lineberger Comprehensive Cancer Center UNC LCCC pilot grant (start-up fund to Z.L.). N.E.S.T. and V.A.P are grateful for NSF Graduate Research Fellowships. A.L. thanks the American Australian Association for a Chevron Fellowship. W.C. thanks G. Bida for assistance with cyclotron operation.
Author information
Authors and Affiliations
Contributions
N.E.S.T. and A.L. contributed equally to the conception and discovery of the project, designed and performed experiments and performed data interpretation for 19F-deoxyfluorination. W.C. established the labelling conditions and conducted the aromatic labelling experiments. V.A.P. assisted in the synthesis and analysis of substrates, products and standards for 19F-deoxyfluorination. Z.W. and Z.H. contributed to the initial labelling design and discussion. D.A.N. and Z.L. conceived and supervised the project and experiments. N.E.S.T., D.A.N. and Z.L. wrote the manuscript with contributions from all the authors.
Corresponding authors
Ethics declarations
Competing interests
Z.L. and D.A.N. have filed US Provisional Patent Application no. 62/812,179 on the technology communicated herein.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1. Time dependence studies for deoxyradiofluorination with arene S2.
All radiochemical yields (RCYs) are calculated by HPLC isolation starting from azeotropically dried [18F]TBAF, decay corrected and represent one experiment unless otherwise noted. See Supplementary Table 5 for more details.
Supplementary information
Supplementary Information
Supplemental Methods, Figs. 1–133, Tables 1–63, spectra and references.
Rights and permissions
About this article
Cite this article
Tay, N.E.S., Chen, W., Levens, A. et al. 19F- and 18F-arene deoxyfluorination via organic photoredox-catalysed polarity-reversed nucleophilic aromatic substitution. Nat Catal 3, 734–742 (2020). https://doi.org/10.1038/s41929-020-0495-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0495-0
This article is cited by
-
Radiochemistry for positron emission tomography
Nature Communications (2023)
-
Preliminary Evaluation of 18F-Labeled Benzylguanidine Analogs as NET Tracers for Myocardial Infarction Diagnosis
Molecular Imaging and Biology (2023)
-
Arene radiofluorination enabled by photoredox-mediated halide interconversion
Nature Chemistry (2022)
-
Closing the gap between 19F and 18F chemistry
EJNMMI Radiopharmacy and Chemistry (2021)