Abstract
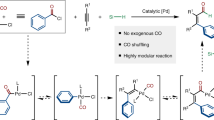
The incorporation of alkynes into organic molecules is one of the most valuable transformations for the formation of C–C bonds and provides a versatile handle for further modifications. The Sonogashira cross-coupling of acetylenes holds a prominent position among the suite of catalytic cross-coupling reactions that are key to modern synthesis. Here we present a method that is complementary to the Sonogashira reaction, demonstrating cross-coupling of lithium acetylides with aryl bromides. The reactions take place under ambient conditions with short reaction times, affording the corresponding aryl acetylenes in good to excellent yields while displaying a remarkable functional group tolerance for an organolithium reaction, allowing the presence of a variety of organolithium-sensitive carbonyl functionalities. This developed cross-coupling methodology offers ample opportunities to access a wide variety of acetylenes, as is illustrated by the facile preparation of key intermediates for chemical biology and optoelectronic materials.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Most data generated or analysed during this study are included in this published article and its Supplementary Information. Actual high-resolution mass spectrometry and gas chromatography–mass spectrometry raw data are available from the corresponding author(s) on reasonable request.
References
Diederich, F., Stang, P. J. & Tykwinski, R. R. Acetylene Chemistry: Chemistry, Biology and Material Science (Wiley-VCH, 2004).
Debets, M. F., van Hest, J. C. M. & Rutjes, F. P. J. T. Bioorthogonal labelling of biomolecules: new functional handles and ligation methods. Org. Biomol. Chem. 11, 6439–6455 (2013).
Bohlmann, F., Burkhardt, H. & Zdero, C. Naturally Occuring Acetylenes (Academic, 1973).
Habrant, D., Rauhala, V. & Koskinen, A. M. P. Conversion of carbonyl compounds to alkynes: general overview and recent developments. Chem. Soc. Rev. 39, 2007–2017 (2010).
Kuang, C., Yang, Q., Senboku, H. & Tokuda, M. Synthesis of (Z)-1-bromo-1-alkenes and terminal alkynes from anti-2,3-dibromoalkanoic acids by microwave-induced reaction. Tetrahedron 61, 4043–4052 (2005).
Biffis, A., Centomo, P., Del Zotto, A. & Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: a critical review. Chem. Rev. 118, 2249–2295 (2018).
Chinchilla, R. & Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 40, 5084–5121 (2011).
Sonogashira, K. in Handbook of Organopalladium Chemistry for Organic Synthesis (ed. Negishi, E.) 493–529 (Wiley, 2002).
Kabalka, G. W. W., Wang, L., Namboodiri, V. & Pagni, R. M. Rapid microwave-enhanced, solventless Sonogashira coupling reaction on alumina. Tetrahedron Lett. 41, 5151–5154 (2000).
Erdélyi, M. & Gogoll, A. Rapid homogeneous-phase Sonogashira coupling reactions using controlled microwave heating. J. Org. Chem. 66, 4165–4169 (2001).
Gholap, A. R. et al. Copper- and ligand-free Sonogashira reaction catalyzed by Pd(0) nanoparticles at ambient conditions under ultrasound irradiation. J. Org. Chem. 70, 4869–4872 (2005).
He, J., Yang, K., Zhao, J. & Cao, S. LiHMDS-promoted palladium-catalyzed Sonogashira cross-coupling of aryl fluorides with terminal alkynes. Org. Lett. 21, 9714–9718 (2019).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Negishi, E. & Xu, C. in Handbook of Organopalladium Chemistry for Organic Synthesis (ed. Negishi, E.) 531–549 (Wiley, 2002).
Umaña, C. A. & Cabezas, J. A. Palladium-catalyzed one-pot conversion of aldehydes and ketones into 4-substituted homopropargyl alcohols and 5-En-3-yn-1-ols. J. Org. Chem. 82, 9505–9514 (2017).
Cabezas, J. A., Poveda, R. R. & Brenes, J. A. One-pot conversion of aldehydes and ketones into 1-substituted and 1,4-disubstituted 1,3-enynes. Synthesis 50, 3307–3321 (2018).
Giannerini, M., Fañanás-Mastral, M. & Feringa, B. L. Direct catalytic cross-coupling of organolithium compounds. Nat. Chem. 5, 667–672 (2013).
Heijnen, D. et al. Oxygen activated, palladium nanoparticle catalyzed, ultrafast cross-coupling of organolithium reagents. Angew. Chem. Int. Ed. 56, 3354–3359 (2017).
Hornillos, V., Giannerini, M., Vila, C., Fañanás-Mastral, M. & Feringa, B. L. Direct catalytic cross-coupling of alkenyllithium compounds. Chem. Sci. 6, 1394–1398 (2015).
Pinxterhuis, E. B., Giannerini, M., Hornillos, V. & Feringa, B. L. Fast, greener and scalable direct coupling of organolithium compounds with no additional solvents. Nat. Commun. 7, 11698 (2016).
Pinxterhuis, E. B., Visser, P., Esser, I., Gualtierotti, J. B. & Feringa, B. L. Fast, efficient and low E-factor one-pot palladium-catalyzed cross-coupling of (hetero)arenes. Angew. Chem. Int. Ed. 57, 9452–9455 (2018).
Huang, X. et al. Expanding Pd-catalyzed C–N bond-forming processes: the first amidation of aryl sulfonates, aqueous amination, and complementarity with Cu-catalyzed reactions. J. Am. Chem. Soc. 125, 6653–6655 (2003).
Negishi, E.-i, Akiyoshi, K. & Takahashi, T. Inhibition of reductive elimination of diorganopalladium species by formation of tetraorganopalladates. J. Chem. Soc. Chem. Commun. 6, 477–478 (1987).
Aufiero, M., Scattolin, T., Proutière, F. & Schoenebeck, F. Air-stable dinuclear iodine-bridged Pd(i) complex—catalyst, precursor or parasite? The additive decides. Systematic nucleophile-activity study and application as precatalyst in cross-coupling. Organometallics 34, 5191–5195 (2015).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Majumdar, K. C. & Chattopadhyay, S. Heterocycles in Natural Product Synthesis (Wiley‐VCH, 2011).
Luisi, R. & Capriati, V. Lithium Compounds in Organic Synthesis—From Fundamentals to Applications (Wiley‐VCH, 2014).
Rappoport, Z. & Marek, I. The Chemistry of Organolithium Compounds (John Wiley & Sons, 2004).
Crabtree, R. H. The Organometallic Chemistry of the Transition Metals (John Wiley & Sons, 2019).
Bordwell, F. G. & Drucker, G. E. Acidities of indene and phenyl-, diphenyl-, and triphenylindenes. J. Org. Chem. 45, 3325–3328 (1980).
Matthews, W. S. et al. Equilibrium acidities of carbon acids. VI. Establishment of an absolute scale of acidities in dimethyl sulfoxide solution. J. Am. Chem. Soc. 97, 7006–7014 (1975).
Fyfe, J. W. B. & Watson, A. J. B. Recent developments in organoboron chemistry: old dogs, new tricks. Chem 3, 31–55 (2017).
Suga, S. & Kitamura, M. 4.17 Asymmetric 1,2-addition of organometallics to carbonyl and imine groups. Compr. Chirality 4, 328–342 (2012).
León, A., Del-Ángel, M., Ávila, J. L. & Delgado, G. in Progress in the Chemistry of Organic Natural Products (eds Kinghorn, A. D. et al.) 127–246 (Springer, 2017).
Oballa, R. M. et al. A generally applicable method for assessing the electrophilicity and reactivity of diverse nitrile-containing compounds. Bioorg. Med. Chem. Lett. 17, 998–1002 (2007).
Nguyen, T. T. T. et al. Chemoselective deprotonative lithiation of azobenzenes: reactions and mechanisms. J. Org. Chem. 79, 2775–2780 (2014).
Haldar, R., Heinke, L. & Wöll, C. Advanced photoresponsive materials using the metal–organic framework approach. Adv. Mater. 32, 1905227 (2019).
Velema, W. A., Szymanski, W. & Feringa, B. L. Photopharmacology: beyond proof of principle. J. Am. Chem. Soc. 136, 2178–2191 (2014).
Dubbaka, S. R., Kienle, M., Mayr, H. & Knochel, P. Copper(i)-mediated oxidative cross-coupling between functionalized alkynyl lithium and aryl magnesium reagents. Angew. Chem. Int. Ed. 46, 9093–9096 (2007).
Nguyen, M. H., O’Brien, K. T. & Smith, A. B. Design, synthesis, and application of polymer-supported silicon-transfer agents for cross-coupling reactions with organolithium reagents. J. Org. Chem. 82, 11056–11071 (2017).
Nagaki, A., Kenmoku, A., Moriwaki, Y., Hayashi, A. & Yoshida, J.-i Cross-coupling in a flow microreactor: space integration of lithiation and murahashi coupling. Angew. Chem. Int. Ed. 49, 7543–7547 (2010).
Quick, M. et al. Photoisomerization dynamics of stiff-stilbene in solution. J. Phys. Chem. B 118, 1389–1402 (2014).
Qin, L., Xu, G.-j, Yao, C. & Xu, Y.-h Conjugated microporous polymer networks with adjustable microstructures for high CO2 uptake capacity and selectivity. Chem. Commun. 52, 12602–12605 (2016).
Carlmark, A., Hawker, C., Hult, A. & Malkoch, M. New methodologies in the construction of dendritic materials. Chem. Soc. Rev. 38, 352–362 (2009).
Brown, C. A. & Yamashita, A. Saline hydrides and superbases in organic reactions. IX. Acetylene zipper. Exceptionally facile contrathermodynamic multipositional isomeriazation of alkynes with potassium 3-aminopropylamide. J. Am. Chem. Soc. 97, 891–892 (1975).
Chowdhury, M. A. & Reissig, H.-U. Syntheses of highly substituted furan and pyrrole derivatives via lithiated 3-aryl-1-methoxyallenes: application to the synthesis of codonopsinine. Synlett 2006, 2383–2386 (2006).
Alemán, J. et al. Enantioselective synthesis of 4-isoxazolines by 1,3-dipolar cycloadditions of nitrones to alkynals catalyzed by fluorodiphenylmethylpyrrolidines. Adv. Synth. Catal. 354, 1665–1671 (2012).
Kivrak, A. & Zora, M. A novel synthesis of 1,2,4-oxadiazoles and isoxazoles. Tetrahedron 70, 817–831 (2014).
Heijnen, D., Helbert, H., Luurtsema, G., Elsinga, P. H. & Feringa, B. L. Synthesis of substituted benzaldehydes via a two-step, one-pot reduction/cross-coupling procedure. Org. Lett. 21, 4087–4091 (2019).
Atherton, J. H. H. & Hall, A. Scale-up—how do we get it right the first time? Chem. Today 29, 47–49 (2011).
Leonard, K. A. et al. 2,4,6-Triarylchalcogenopyrylium dyes related in structure to the antitumor agent AA1 as in vitro sensitizers for the photodynamic therapy of cancer. J. Med. Chem. 42, 3942–3952 (1999).
Jeong, Y., Lee, J. & Ryu, J.-S. Design, synthesis, and evaluation of hinge-binder tethered 1,2,3-triazolylsalicylamide derivatives as Aurora kinase inhibitors. Biorg. Med. Chem. 24, 2114–2124 (2016).
Abrams, J. N., Zhao, Q., Ghiviriga, I. & Minaruzzaman Palladium(ii)-catalyzed enyne cyclization strategies toward the podophyllotoxin ring system. Tetrahedron 68, 423–428 (2012).
Fenenko, L. et al. Electrical properties of 1,4-bis(4-(phenylethynyl)phenylethynyl)benzene and its application for organic light emitting diodes. Chem. Commun. 22, 2278–2280 (2007).
Acknowledgements
B.L.F. acknowledges financial support from the Netherlands Organisation for Scientific Research, the European ResearchCouncil (ERC Advanced Grant 227897), the Royal Netherlands Academy of Arts and Sciences (KNAW), and the Ministry of Education, Culture and Science (Gravitation program 024.601035). S. Crespi is acknowledged for providing (Z)-6,6'-dibromo-2,2',3,3'-tetrahydro-1,1'-bisindenylidene. M. Hansen is acknowledged for providing 1-(4-bromophenyl)-2-phenyldiazene and W. Danowski is acknowledged for providing tetrakis(4-bromophenyl)methane. R. Sneep is acknowledged for performing the high-resolution mass spectrometry.
Author information
Authors and Affiliations
Contributions
H.H., P.V., J.G.H.H. and J.B. performed the experiments. H.H., P.V., J.G.H.H., J.B. and B.L.F. prepared the manuscript. J.B. and B.L.F. designed and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cross-coupling, Organolithium, Sonogashira reaction, Palladium, Acetylene
Supplementary information
Supplementary Information
Supplementary methods, Tables 1–8, references and spectra.
Rights and permissions
About this article
Cite this article
Helbert, H., Visser, P., Hermens, J.G.H. et al. Palladium-catalysed cross-coupling of lithium acetylides. Nat Catal 3, 664–671 (2020). https://doi.org/10.1038/s41929-020-0485-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0485-2