Abstract
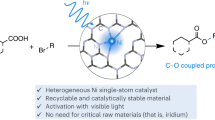
Dual photoredox/nickel-catalysed C–N cross-couplings suffer from low yields for electron-rich aryl halides. The formation of catalytically inactive nickel-black is responsible for this limitation and causes severe reproducibility issues. Here, we demonstrate that catalyst deactivation can be avoided by using a carbon nitride photocatalyst. The broad absorption of the heterogeneous photocatalyst enables wavelength-dependent control of the rate of reductive elimination to prevent nickel-black formation during the coupling of cyclic, secondary amines and aryl halides. A second approach, which is applicable to a broader set of electron-rich aryl halides, is to run the reactions at high concentrations to increase the rate of oxidative addition. Less nucleophilic, primary amines can be coupled with electron-rich aryl halides by stabilizing low-valent nickel intermediates with a suitable additive. The developed protocols enable reproducible, selective C–N cross-couplings of electron-rich aryl bromides and can also be applied for electron-poor aryl chlorides.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Experimental procedures and relevant material and compound characterization data are available in the Supplementary Information. Any other data are available from the authors on reasonable request.
References
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Wolfe, J. P. & Buchwald, S. L. Nickel-catalyzed amination of aryl chlorides. J. Am. Chem. Soc. 119, 6054–6058 (1997).
Ge, S., Green, R. A. & Hartwig, J. F. Controlling first-row catalysts: amination of aryl and heteroaryl chlorides and bromides with primary aliphatic amines catalyzed by a BINAP-ligated single-component Ni(0) complex. J. Am. Chem. Soc. 136, 1617–1627 (2014).
Tassone, J. P., England, E. V., MacQueen, P. M., Ferguson, M. J. & Stradiotto, M. PhPAd-DalPhos: ligand-enabled, nickel-catalyzed cross-coupling of (hetero)aryl electrophiles with bulky primary alkylamines. Angew. Chem. Int. Ed. 58, 2485–2489 (2019).
Kelly, R. A., Scott, N. M., Díez-González, S., Stevens, E. D. & Nolan, S. P. Simple synthesis of CpNi(NHC)Cl complexes (Cp = cyclopentadienyl; NHC = N-heterocyclic carbene). Organometallics 24, 3442–3447 (2005).
Park, N. H., Teverovskiy, G. & Buchwald, S. L. Development of an air-stable nickel precatalyst for the amination of aryl chlorides, sulfamates, mesylates and triflates. Org. Lett. 16, 220–223 (2014).
Kampmann, S. S., Skelton, B. W., Wild, D. A., Koutsantonis, G. A. & Stewart, S. G. An air-stable nickel(0) phosphite precatalyst for primary alkylamine C–N cross-coupling reactions. Eur. J. Org. Chem. 2015, 5995–6004 (2015).
Shields, J. D., Gray, E. E. & Doyle, A. G. A modular, air-stable nickel precatalyst. Org. Lett. 17, 2166–2169 (2015).
McGuire, R. T., Paffile, J. F. J., Zhou, Y. & Stradiotto, M. Nickel-catalyzed C–N cross-coupling of ammonia, (hetero)anilines, and indoles with activated (hetero)aryl chlorides enabled by ligand design. ACS Catal. 9, 9292–9297 (2019).
Li, C. et al. Electrochemically enabled, nickel-catalyzed amination. Angew. Chem. Int. Ed. 56, 13088–13093 (2017).
Kawamata, Y. et al. Electrochemically driven, Ni-catalyzed aryl amination: scope, mechanism and applications. J. Am. Chem. Soc. 141, 6392–6402 (2019).
Lim, C.-H., Kudisch, M., Liu, B. & Miyake, G. M. C–N cross-coupling via photoexcitation of nickel–amine complexes. J. Am. Chem. Soc. 140, 7667–7673 (2018).
Du, Y. et al. Strongly reducing, visible-light organic photoredox catalysts as sustainable alternatives to precious metals. Chem. Eur. J. 23, 10962–10968 (2017).
Caputo, J. A. et al. General and efficient C–C bond forming photoredox catalysis with semiconductor quantum dots. J. Am. Chem. Soc. 139, 4250–4253 (2017).
Liu, Y.-Y., Liang, D., Lu, L.-Q. & Xiao, W.-J. Practical heterogeneous photoredox/nickel dual catalysis for C–N and C–O coupling reactions. Chem. Commun. 55, 4853–4856 (2019).
Ghosh, I. et al. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Science 365, 360–366 (2019).
Corcoran, E. B. et al. Aryl amination using ligand-free Ni(ii) salts and photoredox catalysis. Science 353, 279–283 (2016).
Escobar, R. A. & Johannes, J. A unified and practical method for carbon–heteroatom cross-coupling via nickel/photo dual catalysis. Chem. Eur. J. 26, 5168–5173 (2020).
Kudisch, M., Lim, C.-H., Thordarson, P. & Miyake, G. M. Energy transfer to Ni-amine complexes in dual catalytic, light-driven C–N cross-coupling reactions. J. Am. Chem. Soc. 141, 19479–19486 (2019).
Qi, Z.-H. & Ma, J. Dual role of a photocatalyst: generation of Ni(0) catalyst and promotion of catalytic C–N bond formation. ACS Catal. 8, 1456–1463 (2018).
Wang, C., Cao, S. & Fu, W.-F. A stable dual-functional system of visible-light-driven Ni(ii) reduction to a nickel nanoparticle catalyst and robust in situ hydrogen production. Chem. Commun. 49, 11251–11253 (2013).
Rodríguez, J. L., Valenzuela, M. A., Pola, F., Tiznado, H. & Poznyak, T. Photodeposition of Ni nanoparticles on TiO2 and their application in the catalytic ozonation of 2,4-dichlorophenoxyacetic acid. J. Mol. Catal. A Chem. 353–354, 29–36 (2012).
Indra, A. et al. Nickel as a co-catalyst for photocatalytic hydrogen evolution on graphitic-carbon nitride (sg-CN): what is the nature of the active species? Chem. Commun. 52, 104–107 (2016).
Cavedon, C., Madani, A., Seeberger, P. H. & Pieber, B. Semiheterogeneous dual nickel/photocatalytic (thio)etherification using carbon nitrides. Org. Lett. 21, 5331–5334 (2019).
Pieber, B. et al. Semi-heterogeneous dual nickel/photo-catalysis using carbon nitrides: esterification of carboxylic acids with aryl halides. Angew. Chem. Int. Ed. 58, 9575–9580 (2019).
Zhang, G. et al. Optimizing optical absorption, exciton dissociation, and charge transfer of a polymeric carbon nitride with ultrahigh solar hydrogen production activity. Angew. Chem. Int. Ed. 56, 13445–13449 (2017).
Rosso, C. et al. An oscillatory plug flow photoreactor facilitates semi-heterogeneous dual nickel/carbon nitride photocatalytic C–N couplings. React. Chem. Eng. 5, 597–604 (2020).
Crabtree, R. H. Deactivation in homogeneous transition metal catalysis: causes, avoidance and cure. Chem. Rev. 115, 127–150 (2015).
Molina de la Torre, J. A., Espinet, P. & Albéniz, A. C. Solvent-induced reduction of palladium-aryls, a potential interference in Pd catalysis. Organometallics 32, 5428–5434 (2013).
Zhang, G. et al. Optimizing optical absorption, exciton dissociation, and charge transfer of a polymeric carbon nitride with ultrahigh solar hydrogen production activity. Angew. Chem. Int. Ed. 56, 13445–13449 (2017).
Acknowledgements
We acknowledge the Max Planck Society for generous financial support. S.G. and B.P. thank the International Max Planck Research School on Multiscale Bio-Systems for funding. B.P. and S.R. acknowledge financial support from a Liebig Fellowship of the German Chemical Industry Fund (Fonds der Chemischen Industrie, FCI). B.P. thanks the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC 2008 – 390540038 – UniSysCat) for financial support. We thank our colleagues P.H. Seeberger, J. Malik, K. Gilmore, T. Heil, D. Cruz, H. Runge, R. Pitschke, J. Brandt and K. ten Brummelhuis (all MPIKG) for scientific, technical and analytical support.
Author information
Authors and Affiliations
Contributions
B.P. conceived and directed the research study. B.P., S.G. and S.R. designed all experiments. S.G. performed all synthetic experiments. S.G. and S.R. carried out characterizations of materials and studies on nickel-black formation. S.G. and B.P. wrote the manuscript with contributions from S.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1–9, Figs. 1–41, Tables 1–34 and references.
Rights and permissions
About this article
Cite this article
Gisbertz, S., Reischauer, S. & Pieber, B. Overcoming limitations in dual photoredox/nickel-catalysed C–N cross-couplings due to catalyst deactivation. Nat Catal 3, 611–620 (2020). https://doi.org/10.1038/s41929-020-0473-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0473-6
This article is cited by
-
Poly(heptazine imide) ligand exchange enables remarkable low catalyst loadings in heterogeneous metallaphotocatalysis
Nature Communications (2023)
-
Dihydroquinazolinones as adaptative C(sp3) handles in arylations and alkylations via dual catalytic C–C bond-functionalization
Nature Communications (2022)
-
Red edge effect and chromoselective photocatalysis with amorphous covalent triazine-based frameworks
Nature Communications (2022)
-
Two-photon-absorbing ruthenium complexes enable near infrared light-driven photocatalysis
Nature Communications (2022)
-
Structural transformations of solid electrocatalysts and photocatalysts
Nature Reviews Chemistry (2021)