Abstract
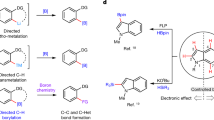
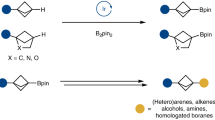
The selective installation of boryl units at less-activated sites, which will facilitate easy access to a range of core structures embedded within bioactive molecules, is a longstanding challenge. Here, we show that catalytic amounts of an earth-abundant Fe(ii)-based complex promote efficient borylations at typically less-reactive positions vicinal (β) to common functional units. Utility is highlighted through the synthesis of drug-like scaffolds and regioconvergent transformation of olefin feedstock to value-added products bearing Cβ–B stereogenic centres. These reactions proceed through tandem alkene isomerization followed by protoboration, and require that the in-situ-generated iron-hydride and iron-boryl catalysts function in synergy. By tuning the two processes of olefin transposition and protoboration, the present Fe-catalysed protocol can provide selective access to 1-boryl-, 2-boryl- or 3-borylalkane isomers. The insights gained from our studies are expected to advance general efforts towards unlocking selective functionalizations at other unactivated sites along the hydrocarbon chain.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available from the corresponding author upon reasonable request. An X-ray crystal structure data file (CCDC reference number 1996790) has been deposited with the Cambridge Crystallographic Data Centre and is available free of charge from www.ccdc.cam.ac.uk/data_request/cif.
References
Hall, D. G. (ed.) Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials Vol. 2 (Wiley-VCH, 2011).
Sandford, C. & Aggarwal, V. K. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 53, 5481–5494 (2017).
Mlynarski, S. N., Schuster, C. H. & Morken, J. P. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 505, 386–390 (2014).
Diaz, D. B. & Yudin, A. K. The versatility of boron in biological target engagement. Nat. Chem. 9, 731–742 (2017).
Baker, S. J. et al. Therapeutic potential of boron-containing compounds. Future Med. Chem. 1, 1275–1288 (2009).
Qiu, F. et al. Recent advances in boron-containing conjugated porous polymers. Polymers 8, 191 (2016).
Yamamoto, E., Maeda, S., Taketsugu, T. & Ito, H. Transition-metal-free boryl substitution using silylboranes and alkoxy bases. Synlett 28, 1258–1267 (2017).
Shintani, R. Recent progress in copper-catalyzed asymmetric allylic substitution reactions using organoboron nucleophiles. Synthesis 48, 1087–1100 (2016).
Hemming, D. et al. Copper-boryl mediated organic synthesis. Chem. Soc. Rev. 47, 7477–7494 (2018).
Semba, K., Fujihara, T., Terao, J. & Tsuji, Y. Copper-catalyzed borylative transformations of non-polar carbon–carbon unsaturated compounds employing borylcopper as an active catalyst species. Tetrahedron 71, 2183–2197 (2015).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev. 110, 890–931 (2010).
Ros, A., Fernández, R. & Lassaletta, J. M. Functional group directed C–H borylation. Chem. Soc. Rev. 43, 3229–3243 (2014).
He, J., Wasa, M., Chan, K. S. L., Shao, Q. & Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 117, 8754–8786 (2017).
Obligacion, J. V. & Chirik, P. J. Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nat. Rev. Chem. 2, 15–34 (2018).
Wei, D. & Darcel, C. Iron catalysis in reduction and hydrometalation reactions. Chem. Rev. 119, 2550–2610 (2019).
Chen, J., Guo, J. & Lu, Z. Recent advances in hydrometallation of alkenes and alkynes via the first row transition metal catalysis. Chin. J. Chem. 36, 1075–1109 (2018).
Liu, Y., Zhou, Y., Wang, H. & Qu, J. FeCl2-catalyzed hydroboration of aryl alkenes with bis(pinacolato)diboron. RSC Adv. 5, 73705–73713 (2015).
Cai, Y. et al. Copper-catalyzed enantioselective markovnikov protoboration of α-olefins enabled by a buttressed N-heterocyclic carbene ligand. Angew. Chem. Int. Ed. 57, 1376–1380 (2018).
Sommer, H., Juliá-Hernández, F., Martin, R. & Marek, I. Walking metals for remote functionalization. ACS Cent. Sci. 4, 153–165 (2018).
Scheuermann, M. L., Johnson, E. J. & Chirik, P. J. Alkene isomerization−hydroboration promoted by phosphine- ligated cobalt catalysts. Org. Lett. 17, 2716–2719 (2015).
Chen, X., Cheng, Z. & Lu, Z. Asymmetric remote C–H borylation of internal alkenes via alkene isomerization. Nat. Commun. 9, 3939 (2018).
Obligacion, J. V. & Chirik, P. J. Bis(imino)pyridine cobalt-catalyzed alkene isomerization−hydroboration: a strategy for remote hydrofunctionalization with terminal selectivity. J. Am. Chem. Soc. 135, 19107–19110 (2013).
Zheng, J., Sortais, J.-B. & Darcel, C. [(NHC)Fe(CO)4] efficient pre-catalyst for selective hydroboration of alkenes. ChemCatChem 6, 763–766 (2014).
Ogawa, T., Ruddy, A. J., Sydora, O. L., Stradiotto, M. & Turculet, L. Cobalt- and iron-catalyzed isomerization−hydroboration of branched alkenes: terminal hydroboration with pinacolborane and 1,3,2-diazaborolanes. Organometallics 36, 417–423 (2017).
Kawamorita, S., Murakami, R., Iwai, T. & Sawamura, M. Synthesis of primary and secondary alkylboronates through site-selective C(sp3)−H activation with silica-supported monophosphine−Ir catalysts. J. Am. Chem. Soc. 135, 2947–2950 (2013).
Larsen, M. A., Cho, S. H. & Hartwig, J. Iridium-catalyzed, hydrosilyl-directed borylation of unactivated alkyl C−H bonds. J. Am. Chem. Soc. 138, 762–765 (2016).
Zhang, S. et al. Delayed catalyst function enables direct enantioselective conversion of nitriles to NH2-amines. Science 364, 45–51 (2019).
Cummings, S. P., Le, T.-N., Fernandez, G. L., Quiambo, L. G. & Stokes, B. J. Tetrahydroxydiboron-mediated palladium-catalyzed transfer hydrogenation and deuteriation of alkenes and alkynes using water as the stoichiometric H or D atom donor. J. Am. Chem. Soc. 138, 6107–6110 (2016).
Neeve, E. C., Geier, S. J., Mkhalid, I. A. I., Westcott, S. A. & Marder, T. B. Diboron(4) compounds: from structural curiosity to synthetic workhorse. Chem. Rev. 116, 9091–9161 (2016).
Nakazawa, H. & Itazaki, M. Fe–H complexes in catalysis. Top. Organomet. Chem. 33, 27–81 (2011).
Mayer, M., Welther, A. & von Wangelin, A. J. Iron-catalyzed isomerizations of olefins. ChemCatChem 3, 1567–1571 (2011).
Crossley, S. W. M., Barabé, F. & Shenvi, R. A. Simple, chemoselective, catalytic olefin isomerization. J. Am. Chem. Soc. 136, 16788–16791 (2014).
Takaya, H. et al. Investigation of organoiron catalysis in kumada–tamao–corriu-type cross-coupling reaction assisted by solution-phase X-ray absorption spectroscopy. Bull. Chem. Soc. Jpn. 88, 410–418 (2015).
Dang, L., Zhao, H., Lin, Z. & Marder, T. B. DFT studies of alkene insertions into Cu−B bonds in copper(I) boryl complexes. Organometallics 26, 2824–2832 (2007).
Zhang, L., Peng, D., Leng, X. & Huang, Z. Iron-catalyzed, atom-economical, chemo- and regioselective alkene hydroboration with pinacolborane. Angew. Chem. Int. Ed. 52, 3676–3680 (2013).
Clémancey, M. et al. Structural insights into the nature of Fe0 and FeI low-valent species obtained upon the reduction of iron salts by aryl grignard reagents. Inorg. Chem. 56, 3834–3848 (2017).
Bedford, R. B. et al. TMEDA in iron-catalyzed kumada coupling: amine adduct versus homoleptic ‘ate’ complex formation. Angew. Chem. Int. Ed. 53, 1804–1808 (2014).
Neidig, M. L. et al. Development and evolution of mechanistic understanding in iron-catalyzed cross-coupling. Acc. Chem. Res. 52, 140–150 (2019).
Sears, J. D., Neate, P. G. N. & Neidig, M. L. Intermediates and mechanism in iron-catalyzed cross-coupling. J. Am. Chem. Soc. 140, 11872–11883 (2018).
Docherty, J. H., Peng, J., Dominey, A. P. & Thomas, S. P. Activation and discovery of earth-abundant metal catalysts using sodium tert-butoxide. Nat. Chem. 9, 595–600 (2017).
Larouche-Gauthier, R., Elford, T. G. & Aggarwal, V. K. Ate complexes of secondary boronic esters as chiral organometallic-type nucleophiles for asymmetric synthesis. J. Am. Chem. Soc. 133, 16794–16797 (2011).
Fang, W. K., Corpuz, E. G. & Chow, K. Disubstituted aryl azetidine derivatives as sphingosine-1-phosphate receptors modulators. US patent WO2015/021109 A1 (2015).
Lu, X. et al. Practical carbon–carbon bond formation from olefins through nickel-catalyzed reductive olefin hydrocarbonation. Nat. Commun. 7, 11129 (2016).
Li, Z., Wang, Z., Zhu, L., Tan, X. & Li, C. Silver-catalyzed radical fluorination of alkylboronates in aqueous solution. J. Am. Chem. Soc. 136, 16439–16443 (2014).
Purohit, A. et al. Quinazoline derivatives as MC-I inhibitors: evaluation of myocardial uptake using positron emission tomography in rat and non-human primate. Bioorg. Med. Chem. Lett. 17, 4882–4885 (2007).
Acknowledgements
This research was supported by the National University of Singapore President’s Assistant Professorship start-up grant no. R-143-000-A50-133 (M.J.K.) and the National Research Foundation, Singapore (NRF) Investigator Award no. NRF-NRF12015-01 (K.P.L.). We thank G. K. Tan for X-ray crystallographic analysis. We also thank N. Yoshikai (Nanyang Technological University), W. Tang (Xi’an Jiaotong University), J. Wang (Dalian Institute of Chemical Physics, Chinese Academy of Sciences) and O. Gutierrez (University of Maryland) for helpful discussions.
Author information
Authors and Affiliations
Contributions
X.Y., H.Z. and L.Q.H.L. developed the method and carried out the mechanistic studies. S.X. performed the EXAFS measurements and analysed the results. Z.C., X.W., L.W. and K.P.L. prepared the materials for EXAFS measurements. M.J.K. directed the investigations and wrote the manuscript with revisions provided by the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 An alternative pathway for alkene chain-walking.
a, Radical clock experiment showing the possibility of radical species formation during the course of hydrometallation. Besides the expected remote protoboration product 7, detectable quantities of 8, likely generated from a hydrometallation/ring rupture/protonolysis/remote protoboration sequence were observed. b, Catalytic cycle for Fe-catalysed C=C bond migration involving hydrogen atom transfer. Conv. and product ratio are determined by GC analysis of unpurfied mixtures. pin, pinacolato; G, substituent; L, ligand.
Extended Data Fig. 2 Operando XANES and EXAFS measurements of Fe-1 and crude reaction mixture.
a, FT-k2χ(R) Fe K-edge EXAFS of the catalyst Fe-1 and reaction mixture at different temperatures. Inset shows an enlarged view of peak II. b, The corresponding Fe K-edge EXAFS spectra. c, The corresponding normalized Fe K-edge XANES spectra. Insets show the enlarged pre-edge peaks and main peaks. RT, room temperature (22 oC).
Extended Data Fig. 3 Temperature- and time-dependent EPR studies.
a, EPR spectra of the reaction mixture with Fe-1 (0.01 mmol), LiOt-Bu (0.30 mmol), B2(pin)2 (0.30 mmol) and 4a (0.20 mmol) in a mixture of DMA (0.16 mL) and toluene (0.34 mL) at various temperatures. b, EPR spectra of the same reaction mixture heated to 100 oC at various times. Test conditions: microwave power (1 mW), central field (317 mT), magnetic width (200 mT), modulation width (1.0 mT), time constant (0.03 s), measurement time (16 min), 120 K.
Supplementary information
Supplementary Information
Supplementary Tables 1–6, Figs. 1–6, methods, Note 1 and references
Crystallographic data 1
Crystallographic data for compound 13
Rights and permissions
About this article
Cite this article
Yu, X., Zhao, H., Xi, S. et al. Site-selective alkene borylation enabled by synergistic hydrometallation and borometallation. Nat Catal 3, 585–592 (2020). https://doi.org/10.1038/s41929-020-0470-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0470-9
This article is cited by
-
Multi-site programmable functionalization of alkenes via controllable alkene isomerization
Nature Chemistry (2023)
-
Structural transformations of solid electrocatalysts and photocatalysts
Nature Reviews Chemistry (2021)
-
γ-Selective C(sp3)–H amination via controlled migratory hydroamination
Nature Communications (2021)