Abstract
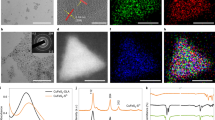
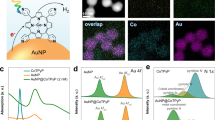
The activation of carbon–fluorine bonds is an industrially and environmentally critical, but energetically challenging, transformation. Here we demonstrate a plasmonic photocatalysis approach to visible-light-driven hydrodefluorination that utilizes aluminium–palladium antenna–reactor heterostructures. Photocatalytic hydrodefluorination of aliphatic carbon–fluorine (C(sp3)–F) bonds in fluoromethane as a model molecule, in the presence of deuterium, results in the selective production of monodeuterated methane with a remarkable photocatalytic efficiency and stability. Analysis of the reaction kinetics reveals a reduction in the apparent reaction barrier and changes to the deuterium reaction order under illumination, which suggests a non-thermal contribution from photogenerated hot carriers to the reaction pathway. Using embedded correlated wavefunction methods, the ground- and excited-state energetics and the role of plasmon excitation in lowering the reaction barrier and modifying the kinetics under illumination are determined. Plasmon-mediated carbon–fluorine bond activation represents a promising potential for applications in high-value chemical transformations, as well as in abatement technologies for the mitigation of anthropogenic polyfluoroorganic compounds.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The atomic coordinates for the surface reactions used in the quantum mechanical simulations are provided as Supplementary Data. The data that support the findings of this paper are available from the corresponding authors upon reasonable request.
Code availability
The modified VASP 5.3.3 code subroutines with embedding implementation and associated Python scripts, and the standalone embedding integral generator code used to transform the embedding potential from Cartesian grid to atomic orbital bases, are available via GitHub, https://github.com/EACcodes/VASPEmbedding and https://github.com/EACcodes/EmbeddingIntegralGenerator, respectively, both under the Mozilla Public License 2.0.
References
Kiplinger, J. L., Richmond, T. G. & Osterberg, C. E. Activation of carbon–fluorine bonds by metal complexes. Chem. Rev. 94, 373–431 (1994).
Richmond, T. G. in Activation of Unreactive Bonds and Organic Synthesis (eds. Murai, S. et al.) 243–269 (Springer, 1999).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Lewandowski, G., Meissner, E. & Milchert, E. Special applications of fluorinated organic compounds. J. Hazard. Mater. 136, 385–391 (2006).
Schaider, L. A. et al. Fluorinated compounds in US fast food packaging. Environ. Sci. Technol. Lett. 4, 105–111 (2017).
Shine, K. P. & Sturges, W. T. Atmospheric science. CO2 is not the only gas. Science 315, 1804–1805 (2007).
Kuehnel, M. F., Lentz, D. & Braun, T. Synthesis of fluorinated building blocks by transition-metal-mediated hydrodefluorination reactions. Angew. Chem. Int. Ed. 52, 3328–3348 (2013).
Ahrens, T., Kohlmann, J., Ahrens, M. & Braun, T. Functionalization of fluorinated molecules by transition-metal-mediated C–F bond activation to access fluorinated building blocks. Chem. Rev. 115, 931–972 (2015).
Fujita, T., Fuchibe, K. & Ichikawa, J. Transition-metal-mediated and -catalyzed C–F bond activation by fluorine elimination. Angew. Chem. Int. Ed. 58, 390–402 (2019).
Amii, H. & Uneyama, K. C−F bond activation in organic synthesis. Chem. Rev. 109, 2119–2183 (2009).
Jones, W. D. Activation of C–F bonds using Cp*2ZrH2: a diversity of mechanisms. Dalton Trans. 2003, 3991–3995 (2003).
Douvris, C. & Ozerov, O. V. Hydrodefluorination of perfluoroalkyl groups using silylium–carborane catalysts. Science 321, 1188–1190 (2008).
Stahl, T., Klare, H. F. T. & Oestreich, M. Main-group Lewis acids for C–F bond activation. ACS Catal. 3, 1578–1587 (2013).
Brongersma, M. L., Halas, N. J. & Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 10, 25–34 (2015).
Christopher, P., Xin, H., Marimuthu, A. & Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 11, 1044–1050 (2012).
Linic, S., Aslam, U., Boerigter, C. & Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 14, 567–576 (2015).
Swearer, D. F. et al. Heterometallic antenna−reactor complexes for photocatalysis. Proc. Natl Acad. Sci. USA 113, 8916–8920 (2016).
Robatjazi, H. et al. Plasmon-induced selective carbon dioxide conversion on Earth-abundant aluminum–cuprous oxide antenna–reactor nanoparticles. Nat. Commun. 8, 27 (2017).
Swearer, D. F. et al. Plasmonic photocatalysis of nitrous oxide into N2 and O2 using aluminum–iridium antenna–reactor nanoparticles. ACS Nano 13, 8076–8086 (2019).
Zhou, L. et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 362, 69–72 (2018).
Li, K. et al. Balancing near-field enhancement, absorption, and scattering for effective antenna–reactor plasmonic photocatalysis. Nano Lett. 17, 3710–3717 (2017).
Wang, F. et al. Plasmonic harvesting of light energy for Suzuki coupling reactions. J. Am. Chem. Soc. 135, 5588–5601 (2013).
Torrens, H. Carbon–fluorine bond activation by platinum group metal complexes. Coord. Chem. Rev. 249, 1957–1985 (2005).
Eisenstein, O., Milani, J. & Perutz, R. N. Selectivity of C–H activation and competition between C–H and C–F bond activation at fluorocarbons. Chem. Rev. 117, 8710–8753 (2017).
Suzuki, N., Fujita, T., Amsharov, K. Y. & Ichikawa, J. Aluminium-mediated aromatic C–F bond activation: regioswitchable construction of benzene-fused triphenylene frameworks. Chem. Commun. 52, 12948–12951 (2016).
Ahrens, M., Scholz, G., Braun, T. & Kemnitz, E. Catalytic hydrodefluorination of fluoromethanes at room temperature by silylium-ion-like surface species. Angew. Chem. Int. Ed. 52, 5328–5332 (2013).
Vol’pin, M. E. et al. Selective hydrogenolysis of the C–F bond. Mendeleev Commun. 1, 118–119 (1991).
Zheng, B. Y. et al. Distinguishing between plasmon-induced and photoexcited carriers in a device geometry. Nat. Commun. 6, 7797 (2015).
Haverkamp, R. G., Metson, J. B., Hyland, M. M. & Welch, B. J. Adsorption of hydrogen fluoride on alumina. Surf. Interface Anal. 19, 139–144 (1992).
Huang, D., Yin, L. & Niu, J. Photoinduced hydrodefluorination mechanisms of perfluorooctanoic acid by the SiC/graphene catalyst. Environ. Sci. Technol. 50, 5857–5863 (2016).
Liu, X., Wang, Z., Zhao, X. & Fu, X. Light induced catalytic hydrodefluorination of perfluoroarenes by porphyrin rhodium. Inorg. Chem. Front. 3, 861–865 (2016).
Khaled, M. B., El Mokadem, R. K. & Weaver, J. D. Hydrogen bond directed photocatalytic hydrodefluorination: overcoming electronic control. J. Am. Chem. Soc. 139, 13092–13101 (2017).
Senaweera, S. M., Singh, A. & Weaver, J. D. Photocatalytic hydrodefluorination: facile access to partially fluorinated aromatics. J. Am. Chem. Soc. 136, 3002–3005 (2014).
Sabater, S., Mata, J. A. & Peris, E. Hydrodefluorination of carbon–fluorine bonds by the synergistic action of a ruthenium–palladium catalyst. Nat. Commun. 4, 1–7 (2013).
Lopez, N., Łodziana, Z., Illas, F. & Salmeron, M. When Langmuir is too simple: H2 dissociation on Pd(111) at high coverage. Phys. Rev. Lett. 93, 146103 (2004).
Spata, V. A. & Carter, E. A. Mechanistic insights into photocatalyzed hydrogen desorption from palladium surfaces assisted by localized surface plasmon resonances. ACS Nano 12, 3512–3522 (2018).
de Jong, G. T. & Bickelhaupt, F. M. Catalytic carbon−halogen bond activation: trends in reactivity, selectivity, and solvation. J. Chem. Theory Comput. 3, 514–529 (2007).
de Jong, G. T. & Bickelhaupt, F. M. Oxidative addition of the fluoromethane C−F bond to Pd. An ab initio benchmark and DFT validation study. J. Phys. Chem. A 109, 9685–9699 (2005).
Li, H., Rivallan, M., Thibault-Starzyk, F., Travert, A. & Meunier, F. C. Effective bulk and surface temperatures of the catalyst bed of FT-IR cells used for in situ and operando studies. Phys. Chem. Chem. Phys. 15, 7321–7327 (2013).
Baffou, G. et al. Photoinduced heating of nanoparticle arrays. ACS Nano 7, 6478–6488 (2013).
Christopher, P., Xin, H. & Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 3, 467–472 (2011).
Kumar, P. V. & Norris, D. J. Tailoring energy transfer from hot electrons to adsorbate vibrations for plasmon-enhanced catalysis. ACS Catal. 7, 8343–8350 (2017).
Frischkorn, C. & Wolf, M. Femtochemistry at metal surfaces: nonadiabatic reaction dynamics. Chem. Rev. 106, 4207–4233 (2006).
Buntin, S. A., Richter, L. J., Cavanagh, R. R. & King, D. S. Optically driven surface reactions: evidence for the role of hot electrons. Phys. Rev. Lett. 61, 1321–1324 (1988).
Vadai, M., Angell, D. K., Hayee, F., Sytwu, K. & Dionne, J. A. In-situ observation of plasmon-controlled photocatalytic dehydrogenation of individual palladium nanoparticles. Nat. Commun. 9, 4658 (2018).
Lynggaard, H., Andreasen, A., Stegelmann, C. & Stoltze, P. Analysis of simple kinetic models in heterogeneous catalysis. Prog. Surf. Sci. 77, 71–137 (2004).
Allian, A. D. et al. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 133, 4498–4517 (2011).
Kale, M. J., Avanesian, T., Xin, H., Yan, J. & Christopher, P. Controlling catalytic selectivity on metal nanoparticles by direct photoexcitation of adsorbate–metal bonds. Nano Lett. 14, 5405–5412 (2014).
McClain, M. J. et al. Aluminum nanocrystals. Nano Lett. 15, 2751–2755 (2015).
Vader, D. T., Viskanta, R. & Incropera, F. P. Design and testing of a high‐temperature emissometer for porous and particulate dielectrics. Rev. Sci. Instrum. 57, 87–93 (1986).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Becke, A. D. & Johnson, E. R. A density-functional model of the dispersion interaction. J. Chem. Phys. 123, 154101 (2005).
Grimme, S., Enrlich, S. & Georigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48, 13115–13118 (1993).
Methfessel, M. & Paxton, A. T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 40, 3616–3621 (1989).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Makov, G. & Payne, M. C. Periodic boundary conditions in ab initio calculations. Phys. Rev. B 51, 4014–4022 (1995).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Blöchl, P. E., Jepsen, O. & Andersen, O. K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 49, 16223–16233 (1994).
Huang, C., Pavone, M. & Carter, E. A. Quantum mechanical embedding theory based on a unique embedding potential. J. Chem. Phys. 134, 154110 (2011).
Govind, N., Wang, Y. A., da Silva, A. J. R. & Carter, E. A. Accurate ab initio energetics of extended systems via explicit correlation embedded in a density functional environment. Chem. Phys. Lett. 295, 129–134 (1998).
Libisch, F., Huang, C. & Carter, E. A. Embedded correlated wavefunction schemes: theory and applications. Acc. Chem. Res. 47, 2768–2775 (2014).
Yu, K., Krauter, C. M., Dieterich, J. M. Carter, E. A. in Fragmentation: Toward Accurate Calculations on Complex Molecular Systems (ed. Gordon, M.) 81–118 (John Wiley & Sons, Inc., 2017).
Dunning, T. H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007–1023 (1989).
Peterson, K. A., Figgen, D., Dolg, M. & Stoll, H. Energy-consistent relativistic pseudopotentials and correlation consistent basis sets for the 4d elements Y–Pd. J. Chem. Phys. 126, 124101 (2007).
Roos, B. O., Taylor, P. R. & Sigbahn, P. E. M. A complete active space SCF method (CASSCF) using a density matrix formulated super-CI approach. Chem. Phys. 48, 157–173 (1980).
Andersson, K., Malmqvist, P. & Roos, B. O. Second‐order perturbation theory with a complete active space self‐consistent field reference function. J. Chem. Phys. 96, 1218–1226 (1992).
Andersson, K., Malmqvist, P. A., Roos, B. O., Sadlej, A. J. & Wolinski, K. Second-order perturbation theory with a CASSCF reference function. J. Phys. Chem. 94, 5483–5488 (1990).
Ghigo, G., Roos, B. O. & Malmqvist, P.-Å. A modified definition of the zeroth-order Hamiltonian in multiconfigurational perturbation theory (CASPT2). Chem. Phys. Lett. 396, 142–149 (2004).
Werner, H. & Meyer, W. A quadratically convergent MCSCF method for the simultaneous optimization of several states. J. Chem. Phys. 74, 5794–5801 (1981).
Forsberg, N. & Malmqvist, P.-Å. Multiconfiguration perturbation theory with imaginary level shift. Chem. Phys. Lett. 274, 196–204 (1997).
Aquilante, F. et al. Molcas 8: new capabilities for multiconfigurational quantum chemical calculations across the periodic table. J. Comput. Chem. 37, 506–541 (2016).
Yu, K., Libisch, F. & Carter, E. A. Implementation of density functional embedding theory within the projector-augmented-wave method and applications to semiconductor defect states. J. Chem. Phys. 143, 102806 (2015).
Johnson, P. B. & Christy, R. W. Optical constants of the noble metals. Phys. Rev. B 6, 4370–4379 (1972).
Palik, E. D. Handbook of Optical Constants of Solids (Academic Press, 1985).
Cheng, F. et al. Epitaxial growth of atomically smooth aluminum on silicon and its intrinsic optical properties. ACS Nano 10, 9852–9860 (2016).
Vasheghani, M. et al. Effect of Al2O3 phases on the enhancement of thermal conductivity and viscosity of nanofluids in engine oil. Heat Mass Transf. 47, 1401–1405 (2011).
Luikov, A. V., Shashkov, A. G., Vasiliev, L. L. & Fraiman, Y. E. Thermal conductivity of porous systems. Int. J. Heat Mass Transf. 11, 117–140 (1968).
Mukhopadhyay, P. & Barua, A. K. Thermal conductivity of hydrogen–helium gas mixtures. Br. J. Appl. Phys. 18, 635–640 (1967).
Acknowledgements
This research was financially supported by the Air Force Office of Scientific Research Multidisciplinary Research Program of the University Research Initiative (MURI FA9550-15-1-0022) (E.A.C., P.N. and N.J.H.), DTRA (HDTRA1-16-1-0042) (N.J.H. and P.N.) and the Welch Foundation under grants C-1220 (N.J.H.) and C-1222 (P.N.). H.R. acknowledges the Postdoctoral Fellowship support in Chemical Science by the Arnold and Mabel Beckman Foundation. E.A.C. acknowledges the High Performance Computing Modernization Program (HPCMP) of the US Department of Defense and Princeton University’s Terascale Infrastructure for Groundbreaking Research in Engineering and Science (TIGRESS) for providing computational resources.
Author information
Authors and Affiliations
Contributions
H.R. and N.J.H. designed the project. H.R. performed the photocatalyst synthesis, carried out photocatalytic experiments and analysed the data. H.R. and L.Z. performed the photocatalyst characterizations. J.L.B. carried out the quantum mechanical calculations. M.Z. performed the electromagnetic simulations. P.C. assisted with interpreting the results. H.R. prepared the initial draft of the manuscript with an assist from J.L.B. All the authors discussed the results and contributed to the final manuscript preparation. E.A.C., P.N. and N.J.H. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–4 and Figs. 1–15.
Supplementary Data
Atomic coordinates for the surface reaction simulations on a palladium Pd(111) slab.
Rights and permissions
About this article
Cite this article
Robatjazi, H., Bao, J.L., Zhang, M. et al. Plasmon-driven carbon–fluorine (C(sp3)–F) bond activation with mechanistic insights into hot-carrier-mediated pathways. Nat Catal 3, 564–573 (2020). https://doi.org/10.1038/s41929-020-0466-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0466-5
This article is cited by
-
Surface plasmon-enhanced photo-driven CO2 hydrogenation by hydroxy-terminated nickel nitride nanosheets
Nature Communications (2023)
-
Plasmon-mediated chemical reactions
Nature Reviews Methods Primers (2023)
-
Reply to: Distinguishing thermal from non-thermal contributions to plasmonic hydrodefluorination
Nature Catalysis (2022)
-
Distinguishing thermal from non-thermal contributions to plasmonic hydrodefluorination
Nature Catalysis (2022)