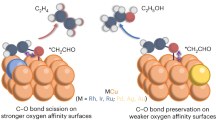
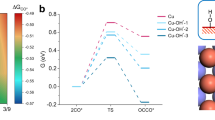
Abstract
Electrocatalytic reduction of CO2 into multicarbon (C2+) products is a highly attractive route for CO2 utilization; however, the yield of C2+ products remains low because of the limited C2+ selectivity at high CO2 conversion rates. Here we report a fluorine-modified copper catalyst that exhibits an ultrahigh current density of 1.6 A cm−2 with a C2+ (mainly ethylene and ethanol) Faradaic efficiency of 80% for electrocatalytic CO2 reduction in a flow cell. The C2–4 selectivity reaches 85.8% at a single-pass yield of 16.5%. We show a hydrogen-assisted C–C coupling mechanism between adsorbed CHO intermediates for C2+ formation. Fluorine enhances water activation, CO adsorption and hydrogenation of adsorbed CO to CHO intermediate that can readily undergo coupling. Our findings offer an opportunity to design highly active and selective CO2 electroreduction catalysts with potential for practical application.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding authors on reasonable request.
References
Olah, G. A., Prakash, G. K. S. & Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 133, 12881–12898 (2011).
Centi, G., Quadrelli, E. A. & Perathoner, S. Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 6, 1711–1731 (2013).
Aresta, M., Dibenedetto, A. & Angelini, A. Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 114, 1709–1742 (2014).
Bushuyev, O. S. et al. What should we make with CO2 and how can we make it? Joule 2, 825–832 (2018).
Yang, H. et al. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 7, 4580–4598 (2017).
Guo, L., Sun, J., Ge, Q. & Tsubaki, N. Recent advances in direct catalytic hydrogenation of carbon dioxide to valuable C2+ hydrocarbons. J. Mater. Chem. A 6, 23244–23262 (2018).
Dokania, A., Ramirez, A., Bavykina, A. & Gascon, J. Heterogeneous catalysis for the valorization of CO2: role of bifunctional processes in the production of chemicals. ACS Energy Lett. 4, 167–176 (2019).
Zhou, W. et al. New horizon in C1 chemistry: breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 48, 3193–3228 (2019).
Porosoff, M. D., Yan, B. & Chen, J. G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: challenges and opportunities. Energy Environ. Sci. 9, 62–73 (2016).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, 350–358 (2019).
Tackett, B. M., Gomez, E. & Chen, J. G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2, 381–386 (2019).
Nielsen, D. U., Hu, X. M., Daasbjerg, K. & Skrydstrup, T. Chemically and electrochemically catalysed conversion of CO2 to CO with follow-up utilization to value-added chemicals. Nat. Catal. 1, 244–254 (2018).
Schouten, K. J. P. et al. A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem. Sci. 2, 1902–1909 (2011).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Gao, D., Arán-Ais, R. M., Jeon, H. S. & Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2, 198–210 (2019).
Qiao, J., Liu, Y., Hong, F. & Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 43, 631–675 (2014).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Loiudice, A. et al. Tailoring copper nanocrystals towards C2 products in electrochemical CO2 reduction. Angew. Chem. Int. Ed. 55, 5789–5792 (2016).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Jiang, K. et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 1, 111–119 (2018).
Zhuang, T. T. et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 1, 421–428 (2018).
Zhou, Y. et al. Dopant-induced electron localization drives CO2 reduction to C2+ hydrocarbons. Nat. Chem. 10, 974–980 (2018).
Ren, D., Ang, B. S. H. & Yeo, B. S. Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived CuxZn Catalysts. ACS Catal. 6, 8239–8247 (2016).
He, J. et al. High-throughput synthesis of mixed-metal electrocatalysts for CO2 reduction. Angew. Chem. Int. Ed. 56, 6068–6072 (2017).
Hoang, T. T. H. et al. Nanoporous copper-silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 140, 5791–5797 (2018).
Morales-Guio, C. G. et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 1, 764–771 (2018).
Varela, A. S., Ju, W., Reier, T. & Strasser, P. Tuning the catalytic activity and selectivity of Cu for CO2 electroreduction in the presence of halides. ACS Catal. 6, 2136–2144 (2016).
Dinh, C. T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Gao, D., Scholten, F. & Roldan Cuenya, B. Improved CO2 electroreduction performance on plasma-activated Cu catalysts via electrolyte design: halide effect. ACS Catal. 7, 5112–5120 (2017).
Lv, J. J. et al. A highly porous copper electrocatalyst for carbon dioxide reduction. Adv. Mater. 30, 1803111 (2018).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Allen, L. C. Electronegativity is the average one-electron energy of the valence-shell electrons in ground-state free atoms. J. Am. Chem. Soc. 111, 9003–9014 (1989).
Gabardo, C. M. et al. Combined high alkalinity and pressurization enable efficient CO2 electroreduction to CO. Energy Environ. Sci. 11, 2531–2539 (2018).
Zhou, H. et al. Fluoroalkyl silane modified silicone rubber/nanoparticle composite: a super durable, robust superhydrophobic fabric coating. Adv. Mater. 24, 2409–2412 (2012).
Chang, X. et al. Tuning Cu/Cu2O interfaces for reduction of carbon dioxide to methanol in aqueous solutions. Angew. Chem. Int. Ed. 57, 15415–15419 (2018).
Totir, G. G., Chottiner, G. S., Gross, C. L. & Scherson, D. A. XPS studies of the chemical and electrochemical behavior of copper in anhydrous hydrogen fluoride. J. Electroanal. Chem. 532, 151–156 (2002).
Lee, W.-H., Byun, J., Cho, S. K. & Kim, J. J. Effect of halides on Cu electrodeposit film: potential-dependent impurity incorporation. J. Electrochem. Soc. 164, 493–497 (2017).
Kau, L. S., Spira-Solomon, D. J., Penner-Hahn, J. E., Hodgson, K. O. & Solomon, E. I. X-ray absorption edge determination of the oxidation state and coordination number of copper. Application to the type 3 site in Rhus vernicifera laccase and its reaction with oxygen. J. Am. Chem. Soc. 109, 6433–6442 (1987).
Huang, Y., Handoko, A. D., Hirunsit, P. & Yeo, B. S. Electrochemical reduction of CO2 using copper single-crystal surfaces: effects of *CO coverage on the selective formation of ethylene. ACS Catal. 7, 1749–1756 (2017).
Sandberg, R. B., Montoya, J. H., Chan, K. & Nørskov, J. K. CO–CO coupling on Cu facets: coverage, strain and field effects. Surf. Sci. 654, 56–62 (2016).
Reuter, K. & Scheffler, M. Composition, structure, and stability of RuO2(110) as a function of oxygen pressure. Phys. Rev. B 65, 035406 (2001).
Montoya, J. H., Peterson, A. A. & Nørskov, J. K. Insights into C–C coupling in CO2 electroreduction on copper electrodes. ChemCatChem 5, 737–742 (2013).
Gao, D. et al. Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte-driven nanostructuring. Angew. Chem. Int. Ed. 58, 17047–17053 (2019).
Gao, S. et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 529, 68–71 (2016).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Subbaraman, R. et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+–Ni(OH)2–Pt interfaces. Science 334, 1256–1260 (2011).
Ma, W. et al. Promoting electrocatalytic CO2 reduction to formate via sulfur-boosting water activation on indium surfaces. Nat. Commun. 10, 892 (2019).
Staszak-Jirkovský, J. et al. Design of active and stable Co–Mo–Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 15, 197–203 (2015).
Cheng, T., Xiao, H. & Goddard, W. A. III Reaction mechanisms for the electrochemical reduction of CO2 to CO and formate on the Cu(100) surface at 298 K from quantum mechanics free energy calculations with explicit water. J. Am. Chem. Soc. 138, 13802–13805 (2016).
Wuttig, A. et al. Tracking a common surface-bound intermediate during CO2-to-fuels catalysis. ACS Cent. Sci. 2, 522–528 (2016).
Krauth, O., Fahsold, G., Magg, N. & Pucci, A. Anomalous infrared transmission of adsorbates on ultrathin metal films: Fano effect near the percolation threshold. J. Chem. Phys. 113, 6330–6333 (2000).
Lu, G. Q. et al. In situ FTIR spectroscopic studies of adsorption of CO, SCN−, and poly (o-phenylenediamine) on electrodes of nanometer thin films of Pt, Pd, and Rh: abnormal infrared effects (AIREs). Langmuir 16, 778–786 (2000).
Lu, X. & Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 6, 6616 (2015).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Lejaeghere, K. et al. Reproducibility in density functional theory calculations of solids. Science 351, aad3000 (2016).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Pack, J. D. & Monkhorst, H. J. Special points for Brillonln-zone integrations—a reply. Phys. Rev. B 16, 1748–1749 (1977).
Wang, H. & Liu, Z. Comprehensive mechanism and structure-sensitivity of ethanol oxidation on platinum: new transition-state searching method for resolving the complex reaction network. J. Am. Chem. Soc. 130, 10996–11004 (2008).
Köhler, L. & Kresse, G. Density functional study of CO on Rh (111). Phys. Rev. B 70, 165405 (2004).
Acknowledgements
This work was supported by the National Key Research and Development Program of the Ministry of Science and Technology of China (no. 2017YFB0602201), the National Natural Science Foundation of China (nos. 21690082, 91545203, 21503176 and 21802110), We thank the staff at the BL14W1 beamline of the Shanghai Synchrotron Radiation Facilities (SSRF) for assistance with the extended X-ray absorption fine structure measurements.
Author information
Authors and Affiliations
Contributions
W.M. and S.X. performed most of the experiments and analysed the experimental data. T.L. and Q.F. performed computational studies and analysed the computational data. J.Y conducted ATR-FTIRS measurements and analysed the results. F.S. and Z.J. conducted XAS measurements and analysed the results. Q.Z. analysed all the data and co-wrote the paper. J.C. guided the computational work, analysed all the data and co-wrote the paper. Y.W. designed and guided the study, and co-wrote the paper. All of the authors discussed the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1–3, Figs. 1–38, Tables 1–8 and References.
Supplementary Data 1
The atomic coordinates of the optimized computational models.
Rights and permissions
About this article
Cite this article
Ma, W., Xie, S., Liu, T. et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat Catal 3, 478–487 (2020). https://doi.org/10.1038/s41929-020-0450-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0450-0
This article is cited by
-
Integrating hydrogen utilization in CO2 electrolysis with reduced energy loss
Nature Communications (2024)
-
Site-selective protonation enables efficient carbon monoxide electroreduction to acetate
Nature Communications (2024)
-
Gas diffusion enhanced electrode with ultrathin superhydrophobic macropore structure for acidic CO2 electroreduction
Nature Communications (2024)
-
Operando formation of highly efficient electrocatalysts induced by heteroatom leaching
Nature Communications (2024)
-
Unraveling the rate-determining step of C2+ products during electrochemical CO reduction
Nature Communications (2024)