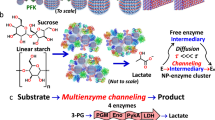
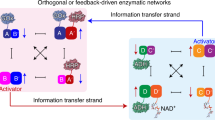
Abstract
Biocatalytic cascades guide complex, efficient and selective intracellular transformations. These unique features originate from the spatial organization of the biocatalysts in confined cellular environments that allow the directional channelling of reaction intermediates across the cells. Here we address efforts directed towards the development of synthetic cell analogues and supramolecular ensembles acting as nano/microenvironments for operating biocatalytic cascades. Multienzyme systems are integrated within metal–organic frameworks, polymersomes, lipid-stabilized microdroplets and hydrogel microparticles acting as cell-like containments. Also, multienzyme systems are spatially positioned on one-dimensional DNA wires, two-dimensional DNA strips or origami tiles, and three-dimensional DNA origami bundles or cages, and specific protein–protein interactions or peptide–protein complexes provide versatile scaffolds for engineering enzyme assemblies. Biocatalytic cascades operating on these scaffolds or in confined nano/microenvironments reveal substantially enhanced reaction yields compared with the analogous diffusional mixtures of the biocomponents. Mechanistic pathways accounting for the enhanced biocatalytic activities and future challenges in developing and applying biocatalytic cascades are presented.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Benetti, E. M., Gunnewiek, M. K., Van Blitterswijk, C. A., Julius Vancso, G. & Moroni, L. Mimicking natural cell environments: design, fabrication and application of bio-chemical gradients on polymeric biomaterial substrates. J. Mater. Chem. B 4, 4244–4257 (2016).
Tu, Y. et al. Mimicking the cell: bio-inspired functions of supramolecular assemblies. Chem. Rev. 116, 2023–2078 (2016).
Salehi-Reyhani, A., Ces, O. & Elani, Y. Artificial cell mimics as simplified models for the study of cell biology. Exp. Biol. Med. 242, 1309–1317 (2017).
Sherman, S. E., Xiao, Q. & Percec, V. Mimicking complex biological membranes and their programmable glycan ligands with dendrimersomes and glycodendrimersomes. Chem. Rev. 117, 6538–6631 (2017).
Trantidou, T. et al. Engineering compartmentalized biomimetic micro- and nanocontainers. ACS Nano 11, 6549–6565 (2017).
Bollhorst, T., Rezwan, K. & Maas, M. Colloidal capsules: nano- and microcapsules with colloidal particle shells. Chem. Soc. Rev. 46, 2091–2126 (2017).
Rideau, E., Dimova, R., Schwille, P., Wurm, F. R. & Landfester, K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem. Soc. Rev. 47, 8572–8610 (2018).
Klermund, L. & Castiglione, K. Polymersomes as nanoreactors for preparative biocatalytic applications: current challenges and future perspectives. Bioprocess Biosyst. Eng. 41, 1233–1246 (2018).
Fanalista, F. et al. Shape and size control of artificial cells for bottom-up biology. ACS Nano 13, 5439–5450 (2019).
Saha, A. et al. In vitro reconstitution of a cell-like environment using liposomes for amyloid beta peptide aggregation and its propagation. Chem. Commun. 49, 6119–6121 (2013).
Xie, J. et al. Oriented assembly of cell-mimicking nanoparticles via a molecular affinity strategy for targeted drug delivery. ACS Nano 13, 5268–5277 (2019).
Kreft, O., Prevot, M., Möhwald, H. & Sukhorukov, G. B. Shell-in-shell microcapsules: a novel tool for integrated, spatially confined enzymatic reactions. Angew. Chem. Int. Ed. 46, 5605–5608 (2007).
Kozlovskaya, V., Baggett, J., Godin, B., Liu, X. & Kharlampieva, E. Hydrogen-bonded multilayers of silk fibroin: from coatings to cell-mimicking shaped microcontainers. ACS Macro Lett. 1, 384–387 (2012).
She, S., Li, Q., Shan, B., Tong, W. & Gao, C. Fabrication of red-blood-cell-like polyelectrolyte microcapsules and their deformation and recovery behavior through a microcapillary. Adv. Mater. 25, 5814–5818 (2013).
Wang, X. et al. Bioinspired approach to multienzyme cascade system construction for efficient carbon dioxide reduction. ACS Catal. 4, 962–972 (2014).
Meng, F. & Zhong, Z. Polymersomes spanning from nano- to microscales: advanced vehicles for controlled drug delivery and robust vesicles for virus and cell mimicking. J. Phys. Chem. Lett. 2, 1533–1539 (2011).
Marguet, M., Sandre, O. & Lecommandoux, S. Polymersomes in ‘gelly’ polymersomes: toward structural cell mimicry. Langmuir 28, 2035–2043 (2012).
Liu, J. et al. DNA-mediated self-organization of polymeric nanocompartments leads to interconnected artificial organelles. Nano Lett. 16, 7128–7136 (2016).
Hu, X. et al. Stimuli-responsive polymersomes for biomedical applications. Biomacromolecules 18, 649–673 (2017).
Liu, X., Formanek, P., Voit, B. & Appelhans, D. Functional cellular mimics for the spatiotemporal control of multiple enzymatic cascade reactions. Angew. Chem. Int. Ed. 56, 16233–16238 (2017).
Larnaudie, S. C., Peyret, A., Beauté, L., Nassoy, P. & Lecommandoux, S. Photopolymerization-induced polymersome rupture. Langmuir 35, 8398–8403 (2019).
Elani, Y., Gee, A., Law, R. V. & Ces, O. Engineering multi-compartment vesicle networks. Chem. Sci. 4, 3332–3338 (2013).
Elani, Y., Solvas, X. C. I., Edel, J. B., Law, R. V. & Ces, O. Microfluidic generation of encapsulated droplet interface bilayer networks (multisomes) and their use as cell-like reactors. Chem. Commun. 52, 5961–5964 (2016).
Karamdad, K. et al. Engineering thermoresponsive phase separated vesicles formed via emulsion phase transfer as a content-release platform. Chem. Sci. 9, 4851–4858 (2018).
Bolognesi, G. et al. Sculpting and fusing biomimetic vesicle networks using optical tweezers. Nat. Commun. 9, 1882 (2018).
Comellas-Aragonès, M. et al. A virus-based single-enzyme nanoreactor. Nat. Nanotechnol. 2, 635–639 (2007).
Patterson, D. P., Schwarz, B., Waters, R. S., Gedeon, T. & Douglas, T. Encapsulation of an enzyme cascade within the bacteriophage P22 virus-like particle. ACS Chem. Biol. 9, 359–365 (2014).
Gao, X. et al. Rapid detection of exosomal microRNAs using virus-mimicking fusogenic vesicles. Angew. Chem. Int. Ed. 58, 8719–8723 (2019).
Chang, F. P., Chen, Y. P. & Mou, C. Y. Intracellular implantation of enzymes in hollow silica nanospheres for protein therapy: cascade system of superoxide dismutase and catalase. Small 10, 4785–4795 (2014).
Godoy-Gallardo, M. et al. Multicompartment artificial organelles conducting enzymatic cascade reactions inside cells. ACS Appl. Mater. Interfaces 9, 15907–15921 (2017).
Rodríguez-Arco, L., Kumar, B. V. V. S. P., Li, M., Patil, A. J. & Mann, S. Modulation of higher-order behaviour in model protocell communities by artificial phagocytosis. Angew. Chem. Int. Ed. 58, 6333–6337 (2019).
Olden, B. R. et al. Cell-templated silica microparticles with supported lipid bilayers as artificial antigen-presenting cells for T cell activation. Adv. Healthc. Mater. 8, 1801188 (2019).
Ikezoe, Y., Washino, G., Uemura, T., Kitagawa, S. & Matsui, H. Autonomous motors of a metal–organic framework powered by reorganization of self-assembled peptides at interfaces. Nat. Mater. 11, 1081–1085 (2012).
Riccò, R. et al. Metal-organic frameworks for cell and virus biology: a perspective. ACS Nano 12, 13–23 (2018).
Ganta, S., Devalapally, H., Shahiwala, A. & Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 126, 187–204 (2008).
Stuart, M. A. C. et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 9, 101–113 (2010).
Dora Tang, T. Y. et al. Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. Nat. Chem. 6, 527–533 (2014).
Ma, G. & Cheng, Q. Vesicular polydiacetylene sensor for colorimetric signaling of bacterial pore-forming toxin. Langmuir 21, 6123–6126 (2005).
Bally, M. et al. Liposome and lipid bilayer arrays towards biosensing applications. Small 6, 2481–2497 (2010).
Yildiz, U. H. et al. Third-party ATP sensing in polymersomes: a label-free assay of enzyme reactions in vesicular compartments. Small 10, 442–447 (2014).
Yu, J. et al. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 17, 733–739 (2017).
Mohammad, M., Razmjou, A., Liang, K., Asadnia, M. & Chen, V. Metal-organic-framework-based enzymatic microfluidic biosensor via surface patterning and biomineralization. ACS Appl. Mater. Interfaces 11, 1807–1820 (2019).
Liu, H. et al. Artificial signal feedback network mimicking cellular adaptivity. J. Am. Chem. Soc. 141, 6458–6461 (2019).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007).
Liu, J., Yang, Q. & Li, C. Towards efficient chemical synthesis via engineering enzyme catalysis in biomimetic nanoreactors. Chem. Commun. 51, 13731–13739 (2015).
Küchler, A., Yoshimoto, M., Luginbühl, S., Mavelli, F. & Walde, P. Enzymatic reactions in confined environments. Nat. Nanotechnol. 11, 409–420 (2016).
Quin, M. B., Wallin, K. K., Zhang, G. & Schmidt-Dannert, C. Spatial organization of multi-enzyme biocatalytic cascades. Org. Biomol. Chem. 15, 4260–4271 (2017).
Sipponen, M. H. et al. Spatially confined lignin nanospheres for biocatalytic ester synthesis in aqueous media. Nat. Commun. 9, 2300 (2018).
Zakharchenko, A., Guz, N., Laradji, A. M., Katz, E. & Minko, S. Magnetic field remotely controlled selective biocatalysis. Nat. Catal. 1, 73–81 (2018).
Nishimura, T. & Akiyoshi, K. Biotransporting biocatalytic reactors toward therapeutic nanofactories. Adv. Sci. 5, 1800801 (2018).
Hwang, E. T. & Lee, S. Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal. 9, 4402–4425 (2019).
Roh, Y. H., Ruiz, R. C. H., Peng, S., Lee, J. B. & Luo, D. Engineering DNA-based functional materials. Chem. Soc. Rev. 40, 5730–5744 (2011).
Wang, F., Liu, X. & Willner, I. DNA switches: from principles to applications. Angew. Chem. Int. Ed. 54, 1098–1129 (2015).
Hu, Y., Cecconello, A., Idili, A., Ricci, F. & Willner, I. Triplex DNA nanostructures: from basic properties to applications. Angew. Chem. Int. Ed. 56, 15210–15233 (2017).
Zhao, Z., Liu, Y. & Yan, H. Organizing DNA origami tiles into larger structures using preformed scaffold frames. Nano Lett. 11, 2997–3002 (2011).
Fu, Y. et al. Single-step rapid assembly of DNA origami nanostructures for addressable nanoscale bioreactors. J. Am. Chem. Soc. 135, 696–702 (2013).
Lin, M. et al. Programmable engineering of a biosensing interface with tetrahedral DNA nanostructures for ultrasensitive DNA detection. Angew. Chem. Int. Ed. 54, 2151–2155 (2015).
He, L. et al. mRNA-initiated, three-dimensional DNA amplifier able to function inside living cells. J. Am. Chem. Soc. 140, 258–263 (2018).
Wang, D., Chai, Y., Yuan, Y. & Yuan, R. Precise regulation of enzyme cascade catalytic efficiency with DNA tetrahedron as scaffold for ultrasensitive electrochemical detection of DNA. Anal. Chem. 91, 3561–3566 (2019).
Peng, P., Du, Y., Zheng, J., Wang, H. & Li, T. Reconfigurable bioinspired framework nucleic acid nanoplatform dynamically manipulated in living cells for subcellular imaging. Angew. Chem. Int. Ed. 58, 1648–1653 (2019).
Tanaka, F. et al. Robust and photocontrollable DNA capsules using azobenzenes. Nano Lett. 10, 3560–3565 (2010).
Cecconello, A., Besteiro, L. V., Govorov, A. O. & Willner, I. Chiroplasmonic DNA-based nanostructures. Nat. Rev. Mater. 2, 17039 (2017).
Xu, Y. et al. Tunable nanoscale cages from self-assembling DNA and protein building blocks. ACS Nano 13, 3545–3554 (2019).
Lu, C. H. & Willner, I. Stimuli-responsive DNA-functionalized nano-/microcontainers for switchable and controlled release. Angew. Chem. Int. Ed. 54, 12212–12235 (2015).
Liao, W. C. & Willner, I. Synthesis and applications of stimuli-responsive DNA-based nano- and micro-sized capsules. Adv. Funct. Mater. 27, 1702732 (2017).
Chen, W. H. et al. Stimuli-responsive nucleic acid-based polyacrylamide hydrogel-coated metal–organic framework nanoparticles for controlled drug release. Adv. Funct. Mater. 28, 1705137 (2018).
Vázquez-González, M. & Willner, I. DNA-responsive SiO2 nanoparticles, metal–organic frameworks, and microcapsules for controlled drug release. Langmuir 34, 14692–14710 (2018).
Liao, W. C., Riutin, M., Parak, W. J. & Willner, I. Programmed pH-responsive microcapsules for the controlled release of CdSe/ZnS quantum dots. ACS Nano 10, 8683–8689 (2016).
Kahn, J. S., Freage, L., Enkin, N., Garcia, M. A. A. & Willner, I. Stimuli-responsive DNA-functionalized metal–organic frameworks (MOFs). Adv. Mater. 29, 1602782 (2017).
Liao, W. C. et al. pH- and ligand-induced release of loads from DNA-acrylamide hydrogel microcapsules. Chem. Sci. 8, 3362–3373 (2017).
Huang, F. et al. Light-responsive and pH-responsive DNA microcapsules for controlled release of loads. J. Am. Chem. Soc. 138, 8936–8945 (2016).
Veetil, A. T. et al. Cell-targetable DNA nanocapsules for spatiotemporal release of caged bioactive small molecules. Nat. Nanotechnol. 12, 1183–1189 (2017).
Chen, W. H. et al. Stimuli-responsive nucleic acid-functionalized metal–organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks. Chem. Sci. 8, 5769–5780 (2017).
Liao, W. C. et al. Adenosine triphosphate-triggered release of macromolecular and nanoparticle loads from aptamer/DNA-cross-linked microcapsules. ACS Nano 9, 9078–9086 (2015).
Liao, W. C. et al. The application of stimuli-responsive VEGF- and ATP-aptamer-based microcapsules for the controlled release of an anticancer drug, and the selective targeted cytotoxicity toward cancer cells. Adv. Funct. Mater. 26, 4262–4273 (2016).
Zhang, Z., Balogh, D., Wang, F. & Willner, I. Smart mesoporous SiO2 nanoparticles for the DNAzyme-induced multiplexed release of substrates. J. Am. Chem. Soc. 135, 1934–1940 (2013).
Zhang, Z., Wang, F., Sohn, Y. S., Nechushtai, R. & Willner, I. Gated mesoporous SiO2 nanoparticles using K+-stabilized G-quadruplexes. Adv. Funct. Mater. 24, 5662–5670 (2014).
Balogh, D., Garcia, M. A. A., Albada, H. B. & Willner, I. Programmed synthesis by stimuli-responsive DNAzyme-modified mesoporous SiO2 nanoparticles. Angew. Chem. Int. Ed. 54, 11652–11656 (2015).
Meng, H. M. et al. DNA dendrimer: an efficient nanocarrier of functional nucleic acids for intracellular molecular sensing. ACS Nano 8, 6171–6181 (2014).
Yang, X. et al. Stimuli-responsive DNA microcapsules for SERS sensing of trace microRNA. ACS Appl. Mater. Interfaces 10, 12491–12496 (2018).
Bhatia, D., Surana, S., Chakraborty, S., Koushika, S. P. & Krishnan, Y. A synthetic icosahedral DNA-based cargo complex for functional in vivo imaging. Nat. Commun. 2, 339 (2011).
Kuzyk, A. et al. Reconfigurable 3D plasmonic metamolecules. Nat. Mater. 13, 862–866 (2014).
Zhou, C., Duan, X. & Liu, N. A plasmonic nanorod that walks on DNA origami. Nat. Commun. 6, 8102 (2015).
Urban, M. J., Zhou, C., Duan, X. & Liu, N. Optically resolving the dynamic walking of a plasmonic walker couple. Nano Lett. 15, 8392–8396 (2015).
Fierobe, H. P. et al. Action of designer cellulosomes on homogeneous versus complex substrates: controlled incorporation of three distinct enzymes into a defined trifunctional scaffoldin. J. Biol. Chem. 280, 16325–16334 (2005).
Lin, J. L., Zhu, J. & Wheeldon, I. Synthetic protein scaffolds for biosynthetic pathway colocalization on lipid droplet membranes. ACS Synth. Biol. 6, 1534–1544 (2017).
Dueber, J. E. et al. Synthetic protein scaffolds provide modular control over metabolic flux. Nat. Biotechnol. 27, 753–759 (2009).
Zakeri, B. et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl Acad. Sci. USA 109, E690–E697 (2012).
Veggiani, G. et al. Programmable polyproteams built using twin peptide superglues. Proc. Natl Acad. Sci. USA 113, 1202–1207 (2016).
Wheeldon, I. et al. Substrate channelling as an approach to cascade reactions. Nat. Chem. 8, 299–309 (2016). A comprehensive review discussing substrate channelling as a mechanistic pathway of cascaded reactions.
Fu, J., Liu, M., Liu, Y. & Yan, H. Spatially-interactive biomolecular networks organized by nucleic acid nanostructures. Acc. Chem. Res. 45, 1215–1226 (2012).
Linko, V. et al. DNA-based enzyme reactors and systems. Nanomaterials 6, 139 (2016).
Rabe, K. S., Müller, J., Skoupi, M. & Niemeyer, C. M. Cascades in compartments: en route to machine-assisted biotechnology. Angew. Chem. Int. Ed. 56, 13574–13589 (2017).
Wang, S. Z. et al. Strategies and perspectives of assembling multi-enzyme systems. Crit. Rev. Biotechnol. 37, 1024–1037 (2017).
Shi, J. et al. Bioinspired construction of multi-enzyme catalytic systems. Chem. Soc. Rev. 47, 4295–4313 (2018).
Liu, Z. et al. Bioinspired dual-enzyme colloidosome reactors for high-performance biphasic catalysis. ACS Appl. Mater. Interfaces 10, 41504–41511 (2018).
Yang, Y. et al. Stepwise degradable nanocarriers enabled cascade delivery for synergistic cancer therapy. Adv. Funct. Mater. 28, 1800706 (2018).
Wang, G. L. et al. A novel photoswitchable enzyme cascade for powerful signal amplification in versatile bioassays. Chem. Commun. 53, 11165–11168 (2017).
Riedel, M., Parak, W. J., Ruff, A., Schuhmann, W. & Lisdat, F. Light as trigger for biocatalysis: photonic wiring of flavin adenine dinucleotide-dependent glucose dehydrogenase to quantum dot-sensitized inverse opal TiO2 architectures via redox polymers. ACS Catal. 8, 5212–5220 (2018).
Lian, X., Chen, Y. P., Liu, T. F. & Zhou, H. C. Coupling two enzymes into a tandem nanoreactor utilizing a hierarchically structured MOF. Chem. Sci. 7, 6969–6973 (2016).
Huang, Y. et al. Growth of Au nanoparticles on 2D metalloporphyrinic metal–organic framework nanosheets used as biomimetic catalysts for cascade reactions. Adv. Mater. 29, 11700102 (2017).
Liu, X. et al. Two-dimensional metal-organic framework/enzyme hybrid nanocatalyst as a benign and self-activated cascade reagent for in vivo wound healing. ACS Nano 13, 5222–5230 (2019).
Liu, X., Qi, W., Wang, Y., Su, R. & He, Z. A facile strategy for enzyme immobilization with highly stable hierarchically porous metal–organic frameworks. Nanoscale 9, 17561–17570 (2017).
Cheng, K., Svec, F., Lv, Y. & Tan, T. Hierarchical micro‐ and mesoporous Zn‐based metal–organic frameworks templated by hydrogels: their use for enzyme immobilization and catalysis of Knoevenagel reaction. Small 15, 1902927 (2019).
Chen, W. H., Vázquez-González, M., Zoabi, A., Abu-Reziq, R. & Willner, I. Biocatalytic cascades driven by enzymes encapsulated in metal–organic framework nanoparticles. Nat. Catal. 1, 689–695 (2018). The study demonstrates the encapsulation of a three-enzyme cascade in porous metal–organic framework nanoparticles.
Liang, J. et al. Peptide-induced super-assembly of biocatalytic metal–organic frameworks for programmed enzyme cascades. Chem. Sci. 10, 7852–7858 (2019).
Vriezema, D. M. et al. Positional assembly of enzymes in polymersome nanoreactors for cascade reactions. Angew. Chem. Int. Ed. 46, 7378–7382 (2007).
Van Dongen, S. F. M., Nallani, M., Cornelissen, J. J. L. M., Nolte, R. J. M. & Van Hest, J. C. M. A three-enzyme cascade reaction through positional assembly of enzymes in a polymersome nanoreactor. Chem. Eur. J. 15, 1107–1114 (2009).
Peters, R. J. R. W. et al. Cascade reactions in multicompartmentalized polymersomes. Angew. Chem. Int. Ed. 53, 146–150 (2014).
Gräfe, D., Gaitzsch, J., Appelhans, D. & Voit, B. Cross-linked polymersomes as nanoreactors for controlled and stabilized single and cascade enzymatic reactions. Nanoscale 6, 10752–10761 (2014).
Rifaie-Graham, O. et al. Wavelength-selective light-responsive DASA-functionalized polymersome nanoreactors. J. Am. Chem. Soc. 140, 8027–8036 (2018).
Elani, Y., Law, R. V. & Ces, O. Vesicle-based artificial cells as chemical microreactors with spatially segregated reaction pathways. Nat. Commun. 5, 5305 (2014). The study describes the preparation of inter-connected lipid-stabilized microdroplets loaded with three enzymes and the operation of a multi-enzyme cascade in the ensemble.
Xiang, B. et al. Self-assembled DNA hydrogel based on enzymatically polymerized DNA for protein encapsulation and enzyme/DNAzyme hybrid cascade reaction. ACS Appl. Mater. Interfaces 8, 22801–22807 (2016).
Tan, H. et al. Heterogeneous multi-compartmental hydrogel particles as synthetic cells for incompatible tandem reactions. Nat. Commun. 8, 663 (2017).
Wang, H. et al. Biomimetic enzyme cascade reaction system in microfluidic electrospray microcapsules. Sci. Adv. 4, eaat2816 (2018).
Wu, Q. et al. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 10, 240 (2019).
Müller, J. & Niemeyer, C. M. DNA-directed assembly of artificial multienzyme complexes. Biochem. Biophys. Res. Commun. 377, 62–67 (2008).
Wilner, O. I., Shimron, S., Weizmann, Y., Wang, Z. & Willner, I. Self-assembly of enzymes on DNA scaffolds: en route to biocatalytic cascades and the synthesis of metallic nanowires. Nano Lett. 9, 2040–2043 (2009).
Wang, Z. G., Wilner, O. I. & Willner, I. Self-assembly of aptamer circular DNA nanostructures for controlled biocatalysis. Nano Lett. 9, 4098–4102 (2009).
Wilner, O. I. et al. Enzyme cascades activated on topologically programmed DNA scaffolds. Nat. Nanotechnol. 4, 249–254 (2009). The study presents the intercommunication of enzyme cascades on 2D DNA strips.
Fu, J., Liu, M., Liu, Y., Woodbury, N. W. & Yan, H. Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures. J. Am. Chem. Soc. 134, 5516–5519 (2012). The study introduces the intercommunication between two enzymes positioned on an origami tile and discusses the separation distance on the efficiency of intermediate chanelling.
Ngo, T. A., Nakata, E., Saimura, M. & Morii, T. Spatially organized enzymes drive cofactor-coupled cascade reactions. J. Am. Chem. Soc. 138, 3012–3021 (2016).
Fu, J. et al. Multi-enzyme complexes on DNA scaffolds capable of substrate channelling with an artificial swinging arm. Nat. Nanotechnol. 9, 531–536 (2014). The study introduces the NAD +-cofactor-mediated communication of enzymes on a spatially organized three-dimensional origami bundle.
Zhao, Z. et al. Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion. Nat. Commun. 7, 10619 (2016).
Hindley, J. W. et al. Light-triggered enzymatic reactions in nested vesicle reactors. Nat. Commun. 9, 1093 (2018).
Hirsch, R., Katz, E. & Willner, I. Magneto-switchable bioelectrocatalysis. J. Am. Chem. Soc. 122, 12053–12054 (2000).
Katz, E., Lioubashevski, O. & Willner, I. Magnetic field effects on bioelectrocatalytic reactions of surface-confined enzyme systems: enhanced performance of biofuel cells. J. Am. Chem. Soc. 127, 3979–3988 (2005).
Guo, W. et al. Switchable bifunctional stimuli-triggered poly-N-isopropylacrylamide/DNA hydrogels. Angew. Chem. Int. Ed. 53, 10134–10138 (2014).
Hu, Y. et al. Reversible modulation of DNA-based hydrogel shapes by internal stress interactions. J. Am. Chem. Soc. 138, 16112–16119 (2016).
Wang, C., Fadeev, M., Vázquez-González, M. & Willner, I. Stimuli-responsive donor-acceptor and DNA-crosslinked hydrogels: application as shape-memory and self-healing materials. Adv. Funct. Mater. 28, 1803111 (2018).
Wang, C. et al. Shape-memory and self-healing functions of DNA-based carboxymethyl cellulose hydrogels driven by chemical or light triggers. Chem. Sci. 9, 7145–7152 (2018).
Liu, X. et al. Chemical and photochemical DNA ‘gears’ reversibly control stiffness, shape-memory, self-healing and controlled release properties of polyacrylamide hydrogels. Chem. Sci. 10, 1008–1016 (2019).
Simmel, F. C., Yurke, B. & Singh, H. R. Principles and applications of nucleic acid strand displacement reactions. Chem. Rev. 119, 6326–6369 (2019).
Seeman, N. C. From genes to machines: DNA nanomechanical devices. Trends Biochem. Sci. 30, 119–125 (2005).
Bath, J. & Turberfield, A. J. DNA nanomachines. Nat. Nanotechnol. 2, 275–284 (2007).
Liu, X., Lu, C. H. & Willner, I. Switchable reconfiguration of nucleic acid nanostructures by stimuli-responsive DNA machines. Acc. Chem. Res. 47, 1673–1680 (2014).
Xin, L., Zhou, C., Yang, Z. & Liu, D. Regulation of an enzyme cascade reaction by a DNA machine. Small 9, 3088–3091 (2013).
Hu, Y. et al. Switchable enzyme/DNAzyme cascades by the reconfiguration of DNA nanostructures. Chem. Eur. J. 20, 16203–16209 (2014). The study introduces the switchable operation of a three-biocatalyst cascade using a DNA tweezer scaffold as driving machinary.
Chen, Y. et al. A synthetic light-driven substrate channeling system for precise regulation of enzyme cascade activity based on DNA origami. J. Am. Chem. Soc. 140, 8990–8996 (2018).
Ke, G. et al. Directional regulation of enzyme pathways through the control of substrate channeling on a DNA origami scaffold. Angew. Chem. Int. Ed. 55, 7483–7486 (2016).
Yang, Y. et al. Programming rotary motions with a hexagonal DNA nanomachine. Chem. Eur. J. 25, 5158–5162 (2019).
Roy, J. J. & Abraham, T. E. Strategies in making cross-linked enzyme crystals. Chem. Rev. 104, 3705–3721 (2004).
Shuvaev, V. V., Dziubla, T., Wiewrodt, R. & Muzykantov, V. R. Streptavidin-biotin crosslinking of therapeutic enzymes with carrier antibodies: nanoconjugates for protection against endothelial oxidative stress. Methods Mol. Biol. 283, 3–19 (2004).
Zhang, Y., Yong, Y., Ge, J. & Liu, Z. Lectin agglutinated multienzyme catalyst with enhanced substrate affinity and activity. ACS Catal. 6, 3789–3795 (2016).
Zhang, G., Quin, M. B. & Schmidt-Dannert, C. Self-assembling protein scaffold system for easy in vitro coimmobilization of biocatalytic cascade enzymes. ACS Catal. 8, 5611–5620 (2018).
Liu, Z., Cao, S., Liu, M., Kang, W. & Xia, J. Self-assembled multienzyme nanostructures on synthetic protein scaffolds. ACS Nano 13, 11343–11352 (2019).
You, C., Myung, S. & Zhang, Y. H. P. Facilitated substrate channeling in a self-assembled trifunctional enzyme complex. Angew. Chem. Int. Ed. 51, 8787–8790 (2012). The study introduces a cellulosome comprising of specific cohesion domains to organize and operate a three-enzyme cascade.
Liu, F., Banta, S. & Chen, W. Functional assembly of a multi-enzyme methanol oxidation cascade on a surface-displayed trifunctional scaffold for enhanced NADH production. Chem. Commun. 49, 3766–3768 (2013).
Qu, J. et al. Synthetic multienzyme complexes, catalytic nanomachineries for cascade biosynthesis in vivo. ACS Nano 13, 9895–9906 (2019). The study describes a versatile concept to genetically engineer enzymes with peptide tags for the organization of supramolecular structures or catcher anchor proteins.
Idan, O. & Hess, H. Engineering enzymatic cascades on nanoscale scaffolds. Curr. Opin. Biotechnol. 24, 606–611 (2013).
Ellis, G. A. et al. Artificial multienzyme scaffolds: Pursuing in vitro substrate channeling with an overview of current progress. ACS Catal. 9, 10812–10869 (2019). A comprehensive review article discussing mechanistic aspects related to cascaded reactions.
Chen, R. et al. Biomolecular scaffolds for enhanced signaling and catalytic efficiency. Curr. Opin. Biotechnol. 28, 59–68 (2014).
Bornscheuer, U. T. et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012).
Idan, O. & Hess, H. Diffusive transport phenomena in artificial enzyme cascades on scaffolds. Nat. Nanotechnol. 7, 769–770 (2012). A study disccusing diffusive intermediate transport phenomena in cascaded multi-enzyme catalysis.
Idan, O. & Hess, H. Origins of activity enhancement in enzyme cascades on scaffolds. ACS Nano 7, 8658–8665 (2013). The study introduces the concept of affinity concentration of the intermediate as pathway to enhance enzyme cascades.
Zhang, Y. & Hess, H. Toward rational design of high-efficiency enzyme cascades. ACS Catal. 7, 6018–6027 (2017).
Lin, J. L., Palomec, L. & Wheeldon, I. Design and analysis of enhanced catalysis in scaffolded multienzyme cascade reactions. ACS Catal. 4, 505–511 (2014).
Lancaster, L., Abdallah, W., Banta, S. & Wheeldon, I. Engineering enzyme microenvironments for enhanced biocatalysis. Chem. Soc. Rev. 47, 5177–5186 (2018).
Fu, J., Liu, M., Liu, Y., Woodbury, N. W. & Yan, H. Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures. J. Am. Chem. Soc. 134, 5516–5519 (2012).
Sweetlove, L. J. & Fernie, A. R. The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation. Nat. Commun. 9, 2136 (2018).
Poshyvailo, L., Von Lieres, E. & Kondrat, S. Does metabolite channeling accelerate enzyme-catalyzed cascade reactions? PLoS ONE 12, e0172673 (2017).
Lin, J. L. & Wheeldon, I. Kinetic enhancements in DNA-enzyme nanostructures mimic the sabatier principle. ACS Catal. 3, 560–564 (2013). A study discussing affinity interaction to enhance intercommunication of enzymes.
Spivey, H. O. & Ovádi, J. Substrate channeling. Methods 19, 306–321 (1999).
Roberts, C. C. & Chang, C. E. A. Modeling of enhanced catalysis in multienzyme nanostructures: effect of molecular scaffolds, spatial organization, and concentration. J. Chem. Theory Comput. 11, 286–292 (2015).
Yong, Y., Pingkai, O., Wu, J. & Liu, Z. A diffusion–reaction model for one-pot synthesis of chemicals with enzyme cascades. ChemCatChem. 12, 528–535 (2020).
Zhang, Y., Tsitkov, S. & Hess, H. Proximity does not contribute to activity enhancement in the glucose oxidase-horseradish peroxidase cascade. Nat. Commun. 7, 13982 (2016).
Medintz, I. Universal tools for biomolecular attachment to surfaces. Nat. Mater. 5, 842 (2006).
Yue, L., Wang, S., Wulf, V. & Willner, I. Stiffness-switchable DNA-based constitutional dynamic network hydrogels for self-healing and matrix-guided controlled chemical processes. Nat. Commun. 10, 4774 (2019).
Tsitkov, S. & Hess, H. Design principles for a compartmentalized enzyme cascade reaction. ACS Catal. 9, 2432–2439 (2019).
Wu, J., Li, S. & Wei, H. Integrated nanozymes: facile preparation and biomedical applications. Chem. Commun. 54, 6520–6530 (2018).
Bugada, L. F., Smith, M. R. & Wen, F. Engineering spatially organized multienzyme assemblies for complex chemical transformation. ACS Catal. 8, 7898–7906 (2018).
Freeman, R., Sharon, E., Tel-Vered, R. & Willner, I. Supramolecular cocaine-aptamer complexes activate biocatalytic cascades. J. Am. Chem. Soc. 131, 5028–5029 (2009).
Katz, E. & Privman, V. Enzyme-based logic systems for information processing. Chem. Soc. Rev. 39, 1835–1857 (2010).
Baron, R., Lioubashevski, O., Katz, E., Niazov, T. & Willner, I. Logic gates and elementary computing by enzymes. J. Phys. Chem. A 110, 8548–8553 (2006).
Wang, F., Lu, C. H. & Willner, I. From cascaded catalytic nucleic acids to enzyme-DNA nanostructures: controlling reactivity, sensing, logic operations, and assembly of complex structures. Chem. Rev. 114, 2881–2941 (2014).
Ohara, T. J., Rajagopalan, R. & Heller, A. “Wired” enzyme electrodes for amperometric determination of glucose or lactate in the presence of interfering substances. Anal. Chem. 66, 2451–2457 (1994).
Trifonov, A. et al. Enzyme-capped relay-functionalized mesoporous carbon nanoparticles: effective bioelectrocatalytic matrices for sensing and biofuel cell applications. ACS Nano 7, 11358–11368 (2013).
Tanner, P., Balasubramanian, V. & Palivan, C. G. Aiding nature’s organelles: artificial peroxisomes play their role. Nano Lett. 13, 2875–2883 (2013).
Acknowledgements
Our research on biocatalytic transformations on confined media is supported by the Volkswagen Foundation, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vázquez-González, M., Wang, C. & Willner, I. Biocatalytic cascades operating on macromolecular scaffolds and in confined environments. Nat Catal 3, 256–273 (2020). https://doi.org/10.1038/s41929-020-0433-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0433-1
This article is cited by
-
Pickering emulsion droplets and solid microspheres acting synergistically for continuous-flow cascade reactions
Nature Catalysis (2024)
-
Efficient NAD+ regeneration facilitated by synergistically intensified charge generation and transfer in fullerene/porphyrin assemblies
Science China Materials (2024)
-
Geometric and defects engineering collaboration for enhanced cascade enzymatic nanoreactors
Nano Research (2024)
-
Multi-compartmental MOF microreactors derived from Pickering double emulsions for chemo-enzymatic cascade catalysis
Nature Communications (2023)
-
Spatiotemporal control for integrated catalysis
Nature Reviews Methods Primers (2023)