Abstract
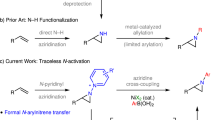
Organocatalytic nitrogen transfer to C=C bonds provides straightforward access to aziridines under mild conditions with low financial and environmental impacts; however, previous methods were typically limited to conjugated C=C bonds (that is, activated olefins), whereas aziridination of isolated C=C bonds (that is, unactivated olefins) remains underexplored. Here we demonstrate a strategy for nitrogen transfer to unactivated olefins by utilizing electron-deficient ketones as catalysts. An oxaziridine intermediate, generated in situ from the ketone catalyst and a nitrogen source, transfers nitrogen to unactivated C=C bonds preferentially over activated C=C bonds. This ‘unusual’ chemoselectivity, as well as the enantioselectivity realized through the use of a chiral ketone catalyst, cannot be achieved by previously developed methods that are based on either organocatalysts or metal catalysts. Moreover, mechanistic studies through modified mass spectrometry allow capture and further investigation of the transient oxaziridine intermediate, establishing its essential role in this nitrogen transfer process.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding authors on reasonable request.
References
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
Kiesewetter, M. K., Shin, E. J., Hedrick, J. L. & Waymouth, R. M. Organocatalysis: opportunities and challenges for polymer synthesis. Macromolecules 43, 2093–2107 (2010).
Alemán, J. & Cabrera, S. Applications of asymmetric organocatalysis in medicinal chemistry. Chem. Soc. Rev. 42, 774–793 (2013).
Zhu, Y., Wang, Q., Cornwall, R. G. & Shi, Y. Organocatalytic asymmetric epoxidation and aziridination of olefins and their synthetic applications. Chem. Rev. 114, 8199–8256 (2014).
Davis, R. L., Stiller, J., Naicker, T., Jiang, H. & Jørgensen, K. A. Asymmetric organocatalytic epoxidations: reactions, scope, mechanisms, and applications. Angew. Chem. Int. Ed. 53, 7406–7426 (2014).
Shi, Y. Organocatalytic asymmetric epoxidation of olefins by chiral ketones. Acc. Chem. Res. 37, 488–496 (2004).
Triandafillidi, I., Tzaras, D. I. & Kokotos, C. G. Green organocatalytic oxidative methods using activated ketones. ChemCatChem 10, 2521–2535 (2018).
Roma, E., Tosi, E., Miceli, M. & Gasperi, T. Asymmetric organocatalytic aziridination: recent advances. Asian J. Org. Chem. 7, 2357–2367 (2018).
Shen, Y.-M., Zhao, M.-X., Xu, J. & Shi, Y. An amine-promoted aziridination of chalcones. Angew. Chem. Int. Ed. 45, 8005–8008 (2006).
Armstrong, A., Baxter, C. A., Lamont, S. G., Pape, A. R. & Wincewicz, R. Amine-promoted, organocatalytic aziridination of enones. Org. Lett. 9, 351–353 (2007).
Vesely, J., Ibrahem, I., Zhao, G.-L., Rios, R. & Córdova, A. Organocatalytic enantioselective aziridination of α,β-unsaturated aldehydes. Angew. Chem. Int. Ed. 46, 778–781 (2007).
Pesciaioli, F. et al. Organocatalytic asymmetric aziridination of enones. Angew. Chem. Int. Ed. 47, 8703–8706 (2008).
De Fusco, C., Fuoco, T., Croce, G. & Lattanzi, A. Noncovalent organocatalytic synthesis of enantioenriched terminal aziridines with a quaternary stereogenic center. Org. Lett. 14, 4078–4081 (2012).
Halskov, K. S., Naicker, T., Jensen, M. E. & Jørgensen, K. A. Organocatalytic asymmetric remote aziridination of 2,4-dienals. Chem. Commun. 49, 6382–6384 (2013).
Frankowski, S. et al. Organocatalytic synthesis of cis-2,3-aziridine aldehydes by a postreaction isomerization. Org. Lett. 19, 5000–5003 (2017).
Yoshimura, A., Middleton, K. R., Zhu, C., Nemykin, V. N. & Zhdankin, V. V. Hypoiodite-mediated metal-free catalytic aziridination of alkenes. Angew. Chem. Int. Ed. 51, 8059–8062 (2012).
Jeong, J. U., Tao, B., Sagasser, I., Henniges, H. & Sharpless, K. B. Bromine-catalyzed aziridination of olefins. A rare example of atom-transfer redox catalysis by a main group element. J. Am. Chem. Soc. 120, 6844–6845 (1998).
Jat, J. L. et al. Direct stereospecific synthesis of unprotected N-H and N-Me aziridines from olefins. Science 343, 61–65 (2014).
Ma, Z., Zhou, Z. & Kürti, L. Direct and stereospecific synthesis of N-H and N-alkyl aziridines from unactivated olefins using hydroxylamine-O-sulfonic acids. Angew. Chem. Int. Ed. 56, 9886–9890 (2017).
Sabir, S., Pandey, C. B., Yadav, A. K., Tiwari, B. & Jat, J. L. Direct N-H/N-Me aziridination of unactivated olefins using O-(sulfonyl)hydroxylamines as aminating agents. J. Org. Chem. 83, 12255–12260 (2018).
Gao, H. et al. Rapid heteroatom transfer to arylmetals utilizing multifunctional reagent scaffolds. Nat. Chem. 9, 681–688 (2017).
Zhou, Z., Ma, Z., Behnke, N. E., Gao, H. & Kürti, L. Non-deprotonative primary and secondary amination of (hetero)arylmetals. J. Am. Chem. Soc. 139, 115–118 (2017).
Andreae, S. & Schmitz, E. Electrophilic aminations with oxaziridines. Synthesis 1991, 327–341 (1991).
Williamson, K. S., Michaelis, D. J. & Yoon, T. P. Advances in the chemistry of oxaziridines. Chem. Rev. 114, 8016–8036 (2014).
Washington, I., Houk, K. N. & Armstrong, A. Strategies for the design of organic aziridination reagents and catalysts: transition structures for alkene aziridinations by NH transfer. J. Org. Chem. 68, 6497–6501 (2003).
Wallace, R. G. Hydroxylamine-O-sulfonic acid — a versatile synthetic reagent. Aldrichimica Acta 13, 3–11 (1980).
Colomer, I., Chamberlain, A. E. R., Haughey, M. B. & Donohoe, T. J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 1, 0088 (2017).
Gershenzon, J. & Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 3, 408–414 (2007).
Davies, H. M. L. & Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Iacobucci, C., Reale, S. & De Angelis, F. Elusive reaction intermediates in solution explored by ESI-MS: reverse periscope for mechanistic investigations. Angew. Chem. Int. Ed. 55, 2980–2993 (2016).
Acknowledgements
L.K. acknowledges financial support from Rice University, the National Institutes of Health (R01 grant no. GM-114609-04), the National Science Foundation (CAREER:SusChEM grant no. CHE-1546097) and the Robert A. Welch Foundation (grant no. C-1764). X.Z. is grateful for financial support from the Shenzhen STIC (grant no. JCYJ20170412150343516) and the Shenzhen San-Ming Project (grant no. SZSM201809085). D.H.E. thanks BYU and the Fulton Supercomputing Lab.
Author information
Authors and Affiliations
Contributions
Q.-Q.C. and L.K. conceived this work and designed the synthetic studies; Q.-Q.C. conducted the synthetic studies with assistance from Z.Z.; X.Z. and D.H.E. designed the mechanistic studies; H.J. and D.H.E. conducted the mechanistic studies with assistance from Q.-Q.C. and J.H.S.; Q.-Q.C. and L.K. prepared the manuscript with input from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Suplementary information
Supplementary Information
Supplementary Notes 1–3, Tables 1–4, methods, Figs. 1–7 and references.
Supplementary Data 1
Cartesian coordinates and energies for calculated structures.
Rights and permissions
About this article
Cite this article
Cheng, QQ., Zhou, Z., Jiang, H. et al. Organocatalytic nitrogen transfer to unactivated olefins via transient oxaziridines. Nat Catal 3, 386–392 (2020). https://doi.org/10.1038/s41929-020-0430-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0430-4
This article is cited by
-
N-Aminopyridinium reagents as traceless activating groups in the synthesis of N-Aryl aziridines
Nature Communications (2022)
-
Caryophyllene and caryophyllene oxide: a variety of chemical transformations and biological activities
Chemical Papers (2022)
-
Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication
Nature (2021)