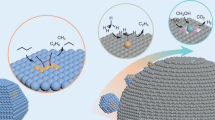
Abstract
Reducing the size of metal nanoparticles down to the single-atom level has been actively pursued to maximize the use of precious metals. Recently, single-atom catalysts, in which all the metal atoms are isolated on a support with 100% dispersion, have received much attention. However, the lack of ensemble sites prevents valuable surface reactions that require metal proximity to occur. Here, we present metal (Pt, Pd and Rh) ensemble catalysts with 100% dispersion and a reduced metallic surface state. More specifically, nanoceria particles were anchored on Al3+penta sites of activated γ-alumina, and then metal was deposited and reduced. The ensemble catalysts are highly durable: their structure was maintained even after hydrothermal ageing at 900 °C for 24 h or after long-term reaction. These catalysts have superior activity and durability for three-way catalytic reactions and can provide insights beyond single-atom catalysts for heterogeneous catalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author on reasonable request.
References
Wang, A. Q., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Yang, X. F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Liu, L. C. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Guczi, L. et al. Gold nanoparticles deposited on SiO2/Si(100): correlation between size, electron structure, and activity in CO oxidation. J. Am. Chem. Soc. 125, 4332–4337 (2003).
Haneda, M., Watanabe, T., Kamiuchi, N. & Ozawa, M. Effect of platinum dispersion on the catalytic activity of Pt/Al2O3 for the oxidation of carbon monoxide and propene. Appl. Catal. B 142, 8–14 (2013).
Bonanni, S., Ait-Mansour, K., Harbich, W. & Brune, H. Reaction-induced cluster ripening and initial size-dependent reaction rates for CO oxidation on Ptn/TiO2(110)-(1x1). J. Am. Chem. Soc. 136, 8702–8707 (2014).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Lin, L. L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017).
Zhang, Z. L. et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 8, 16100 (2017).
Lin, J. et al. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 135, 15314–15317 (2013).
Yan, H. et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 137, 10484–10487 (2015).
Kwon, Y., Kim, T. Y., Kwon, G., Yi, J. & Lee, H. Selective activation of methane on single-atom catalyst of rhodium dispersed on zirconia for direct conversion. J. Am. Chem. Soc. 139, 17694–17699 (2017).
Peterson, E. J. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014).
Choi, C. H. et al. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun. 7, 10922 (2016).
Kim, J. et al. Highly durable platinum single-atom alloy catalyst for electrochemical reactions. Adv. Energy Mater. 8, 1701476 (2018).
Yang, S., Kim, J., Tak, Y. J., Soon, A. & Lee, H. Single-atom catalyst of platinum supported on titanium nitride for selective electrochemical reactions. Angew. Chem. Int. Ed. 55, 2058–2062 (2016).
Moses-DeBusk, M. et al. CO oxidation on supported single Pt atoms: experimental and ab initio density functional studies of CO interaction with Pt atom on θ-Al2O3(010) surface. J. Am. Chem. Soc. 135, 12634–12645 (2013).
Therrien, A. J. et al. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 1, 192–198 (2018).
Shan, J. J., Li, M. W., Allard, L. F., Lee, S. S. & Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 551, 605–608 (2017).
Wang, L. B. et al. Atomic-level insights in optimizing reaction paths for hydroformylation reaction over Rh/CoO single-atom catalyst. Nat. Commun. 7, 14036 (2016).
Kyriakou, G. et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335, 1209–1212 (2012).
Lucci, F. R. et al. Selective hydrogenation of 1,3-butadiene on platinum-copper alloys at the single-atom limit. Nat. Commun. 6, 8550 (2015).
Bae, J., Kim, J., Jeong, H. & Lee, H. CO oxidation on SnO2 surfaces enhanced by metal doping. Catal. Sci. Technol. 8, 782–789 (2018).
Jeong, H. et al. Fully dispersed Rh ensemble catalyst to enhance low-temperature activity. J. Am. Chem. Soc. 140, 9558–9565 (2018).
Gabelnick, A. M., Capitano, A. T., Kane, S. M., Gland, J. L. & Fischer, D. A. Propylene oxidation mechanisms and intermediates using in situ soft x-ray fluorescence methods on the Pt(111) surface. J. Am. Chem. Soc. 122, 143–149 (2000).
Lee, I., Delbecq, F., Morales, R., Albiter, M. A. & Zaera, F. Tuning selectivity in catalysis by controlling particle shape. Nat. Mater. 8, 132–138 (2009).
An, K., Alayoglu, S., Musselwhite, N., Na, K. & Somorjai, G. A. Designed catalysts from Pt nanoparticles supported on macroporous oxides for selective isomerization of n-hexane. J. Am. Chem. Soc. 136, 6830–6833 (2014).
Kliewer, C. J. et al. Furan hydrogenation over Pt(111) and Pt(100) single-crystal surfaces and Pt nanoparticles from 1 to 7 nm: a kinetic and sum frequency generation vibrational spectroscopy study. J. Am. Chem. Soc. 132, 13088–13095 (2010).
An, K. & Somorjai, G. A. Size and shape control of metal nanoparticles for reaction selectivity in catalysis. ChemCatChem 4, 1512–1524 (2012).
Chia, M. et al. Selective hydrogenolysis of polyols and cyclic ethers over bifunctional surface sites on rhodium-rhenium catalysts. J. Am. Chem. Soc. 133, 12675–12689 (2011).
Wang, G. H. et al. Platinum-cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural. Nat. Mater. 13, 293–300 (2014).
Lykhach, Y. et al. Counting electrons on supported nanoparticles. Nat. Mater. 15, 284–288 (2016).
Yang, S., Tak, Y. J., Kim, J., Soon, A. & Lee, H. Support effects in single-atom platinum catalysts for electrochemical oxygen reduction. ACS Catal. 7, 1301–1307 (2017).
Wei, H. S. et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 5, 5634 (2014).
Zhang, S. R. et al. Catalysis on singly dispersed Rh atoms anchored on an inert support. ACS Catal. 8, 110–121 (2018).
Kwak, J. H. et al. Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on γ-Al2O3. Science 325, 1670–1673 (2009).
Kwak, J. H., Hu, J. Z., Kim, D. H., Szanyi, J. & Peden, C. H. F. Penta-coordinated Al3+ ions as preferential nucleation sites for BaO on γ-Al2O3: an ultra-high-magnetic field 27Al MAS NMR study. J. Catal. 251, 189–194 (2007).
Bond, G. C. Heterogeneous Catalysis: Principles and Applications 2nd edn (Oxford Univ. Press, 1987).
Ertl, G., Knözinger, H., Schuth, F. & Weitkamp, J. Handbook of Heterogeneous Catalysis 2nd edn (Wiley, 2008).
Ding, K. et al. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Jeong, H., Bae, J., Han, J. W. & Lee, H. Promoting effects of hydrothermal treatment on the activity and durability of Pd/CeO2 catalysts for CO oxidation. ACS Catal. 7, 7097–7105 (2017).
Lang, R. et al. Hydroformylation of olefins by a rhodium single-atom catalyst with activity comparable to RhCl(PPh3)3. Angew. Chem. Int. Ed. 55, 16054–16058 (2016).
Avakyan, L. A. et al. Evolution of the atomic structure of ceria-supported platinum nanocatalysts: formation of single layer platinum oxide and Pt-O-Ce and Pt-Ce linkages. J. Phys. Chem. C 120, 28057–28066 (2016).
Zhang, C. C. & Lin, J. Visible-light induced oxo-bridged ZrIV-O-CeIII redox centre in tetragonal ZrO2-CeO2 solid solution for degradation of organic pollutants. Phys. Chem. Chem. Phys. 13, 3896–3905 (2011).
Lustemberg, P. G. et al. Room-temperature activation of methane and dry re-forming with CO2 on Ni-CeO2(111) surfaces: effect of Ce3+ sites and metal-support interactions on C-H bond cleavage. ACS Catal. 6, 8184–8191 (2016).
USDRIVE Future Automotive Aftertreatment Solutions: the 150 °C Challenge Workshop Report (Pacific Northwest National Laboratory, 2012).
USDRIVE Advanced Combustion and Emission Control Technical Team Roadmap; Driving Research and Innovation for Vehicle Efficiency and Energy Sustainability (US Department of Energy, 2013).
Lupescu, J. A. et al. Pd model catalysts: effect of aging duration on lean redispersion. Appl. Catal. B 185, 189–202 (2016).
Kwak, J. H. et al. Role of pentacoordinated Al3+ ions in the high temperature phase transformation of γ-Al2O3. J. Phys. Chem. C 112, 9486–9492 (2008).
Takeguchi, T. et al. Determination of dispersion of precious metals on CeO2-containing supports. Appl. Catal. A 293, 91–96 (2005).
Gatica, J. M. et al. Rhodium dispersion in a Rh/Ce0.68Zr0.32O2 catalyst investigated by HRTEM and H2 chemisorption. J. Phys. Chem. B 104, 4667–4672 (2000).
Lu, Z. S. & Yang, Z. X. Interfacial properties of NM/CeO2(111) (NM = noble metal atoms or clusters of Pd, Pt and Rh): a first principles study. J. Phys. Condens. Matter 22, 475003 (2010).
Acknowledgements
This research was supported by the National Research Foundation of Korea (grant numbers NRF-2016R1A5A1009592 and 2018R1A2A2A05018849). The experiments at PLS were supported in part by MSIP and POSTECH.
Author information
Authors and Affiliations
Contributions
H.J. and H.L. conceived the project. H.J. designed the synthesis of the catalysts. O.K. and J.K. performed the computational calculation. H.J., B.-S.K. and J.B. carried out the characterizations and catalytic reactions. S.S. helped with the X-ray absorption analysis, and H.-E.K. helped with the transmission electron microscopy analysis. H.J., O.K., J.K. and H.L. wrote the manuscript. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31, Tables 1–13 and references.
Supplementary Dataset 1
Atomic coordinates of the optimized DFT models
Rights and permissions
About this article
Cite this article
Jeong, H., Kwon, O., Kim, BS. et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat Catal 3, 368–375 (2020). https://doi.org/10.1038/s41929-020-0427-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0427-z
This article is cited by
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)
-
Tripodal Pd metallenes mediated by Nb2C MXenes for boosting alkynes semihydrogenation
Nature Communications (2023)
-
A single site ruthenium catalyst for robust soot oxidation without platinum or palladium
Nature Communications (2023)
-
Dynamic and reversible transformations of subnanometre-sized palladium on ceria for efficient methane removal
Nature Catalysis (2023)
-
Selective dissolution to synthesize densely populated Pt single atom catalyst
Nano Research (2023)