Abstract
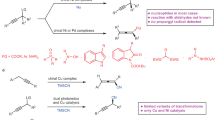
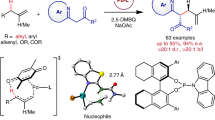
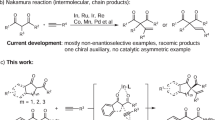
The asymmetric one-step net addition of unactivated propargylic C–H bonds to aldehydes leads to an atom-economic construction of versatile chiral homopropargylic alcohols, but has not yet been realized. Here we show its implementation in an intramolecular manner under mild reaction conditions. This chemistry—via cooperative gold catalysis enabled by a chiral bifunctional phosphine ligand—achieves asymmetric catalytic deprotonation of propargylic C–H (pKa > 30) by a tertiary amine group (pKa ≈ 10) of the ligand in the presence of much more acidic aldehydic α-hydrogens (pKa ≈ 17). The reaction exhibits a broad scope and readily accommodates various functional groups. The cyclopentane/cyclohexane-fused homopropargylic alcohol products are formed with excellent enantiomeric excesses and high trans-selectivities with or without a preexisting substrate chiral centre. Density functional theory studies of the reaction support the conceived reaction mechanism and the calculated energetics corroborate the observed stereoselectivity and confirm additional metal–ligand cooperation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Experimental procedures, characterization of compounds and DFT calculations are available in the Supplementary Information. The X-ray diffraction data for 11, 6p and (S)-L3AuCl are deposited to the Cambridge Crystallographic Data Centre (CCDC) with the reference numbers 1988012, 1988013 and 1988482, respectively. All data are available from the authors on reasonable request.
References
Ding, C.-H. & Hou, X.-L. Catalytic asymmetric propargylation. Chem. Rev. 111, 1914–1937 (2011).
Yang, X., Kalita, S. J., Maheshuni, S. & Huang, Y.-Y. Recent advances on transition-metal-catalyzed asymmetric tandem reactions with organoboron reagents. Coord. Chem. Rev. 392, 35–48 (2019).
Alvarez, L. X., Christ, M. L. & Sorokin, A. B. Selective oxidation of alkenes and alkynes catalyzed by copper complexes. Appl. Catal. A 325, 303–308 (2007).
Cheng, D. & Bao, W. Propargylation of 1,3-dicarbonyl compounds with 1,3-diarylpropynes via oxidative cross-coupling between sp3 C–H and sp3 C–H. J. Org. Chem. 73, 6881–6883 (2008).
Grigg, R. D., Rigoli, J. W., Pearce, S. D. & Schomaker, J. M. Synthesis of propargylic and allenic carbamates via the C–H amination of alkynes. Org. Lett. 14, 280–283 (2012).
Wang, T., Zhou, W., Yin, H., Ma, J.-A. & Jiao, N. Iron-facilitated oxidative dehydrogenative C–O bond formation by propargylic C–H functionalization. Angew. Chem. Int. Ed. 51, 10823–10826 (2012).
Lu, H., Li, C., Jiang, H., Lizardi, C. L. & Zhang, X. P. Chemoselective amination of propargylic C(sp3)–H bonds by cobalt(ii)-based metalloradical catalysis. Angew. Chem. Int. Ed. 53, 7028–7032 (2014).
Fernández-Salas, J. A., Eberhart, A. J. & Procter, D. J. Metal-free CH–CH-type cross-coupling of arenes and alkynes directed by a multifunctional sulfoxide group. J. Am. Chem. Soc. 138, 790–793 (2016).
Hu, G., Xu, J. & Li, P. Sulfur mediated propargylic C–H alkylation of alkynes. Org. Chem. Front. 5, 2167–2170 (2018).
Ju, M. et al. Silver-catalyzed enantioselective propargylic C–H bond amination through rational ligand design. J. Am. Chem. Soc. 142, 12930–12936 (2020).
Wang, Y., Zhu, J., Durham, A. C., Lindberg, H. & Wang, Y.-M. α-C–H Functionalization of π-bonds using iron complexes: catalytic hydroxyalkylation of alkynes and alkenes. J. Am. Chem. Soc. 141, 19594–19599 (2019).
Li, T. & Zhang, L. Bifunctional biphenyl-2-ylphosphine ligand enables tandem gold-catalyzed propargylation of aldehyde and unexpected cycloisomerization. J. Am. Chem. Soc. 140, 17439–17443 (2018).
Wang, Z., Wang, Y. & Zhang, L. Soft propargylic deprotonation: designed ligand enables Au-catalyzed isomerization of alkynes to 1,3-dienes. J. Am. Chem. Soc. 136, 8887–8890 (2014).
Wang, Z., Ying, A., Fan, Z., Hervieu, C. & Zhang, L. Tertiary amino group in cationic gold catalyst: tethered frustrated lewis pairs that enable ligand-controlled regiodivergent and stereoselective isomerizations of propargylic esters. ACS Catal. 7, 3676–3680 (2017).
Li, X., Ma, X., Wang, Z., Liu, P.-N. & Zhang, L. Bifunctional phosphine ligand enabled gold-catalyzed alkynamide cycloisomerization: access to electron-rich 2-aminofurans and their Diels–Alder adducts. Angew. Chem. Int. Ed. 58, 17180–17184 (2019).
Li, X., Wang, Z., Ma, X., Liu, P.-N. & Zhang, L. Designed bifunctional phosphine ligand-enabled gold-catalyzed isomerizations of ynamides and allenamides: stereoselective and regioselective formation of 1-amido-1,3-dienes. Org. Lett. 19, 5744–5747 (2017).
Wang, H., Li, T., Zheng, Z. & Zhang, L. Efficient synthesis of α-allylbutenolides from allyl ynoates via tandem ligand-enabled Au(i) catalysis and the Claisen rearrangement. ACS Catal. 9, 10339–10342 (2019).
Li, T., Yang, Y., Li, B., Bao, X. & Zhang, L. Gold-catalyzed silyl-migrative cyclization of homopropargylic alcohols enabled by bifunctional biphenyl-2-ylphosphine and DFT studies. Org. Lett. 21, 7791–7794 (2019).
Cheng, X., Wang, Z., Quintanilla, C. D. & Zhang, L. Chiral bifunctional phosphine ligand enabling gold-catalyzed asymmetric isomerization of alkyne to allene and asymmetric synthesis of 2,5-dihydrofuran. J. Am. Chem. Soc. 141, 3787–3791 (2019).
Schnabel, C. et al. Total synthesis of natural and non-natural Δ5,6Δ12,13-jatrophane diterpenes and their evaluation as MDR modulators. J. Org. Chem. 76, 512–522 (2011).
Dahlmann, H. A., McKinney, A. J., Santos, M. P. & Davis, L. O. Organocatalyzed intramolecular carbonyl–ene reactions. Molecules 21, 713 (2016).
Amarasinghe, K. K. D. & Montgomery, J. Enantioselective total synthesis of (+)-testudinariol a using a new nickel-catalyzed allenyl aldehyde cyclization. J. Am. Chem. Soc. 124, 9366–9367 (2002).
Zhou, J., Yang, M., Akdag, A. & Schneller, S. W. C-4′ Truncated carbocyclic formycin derivatives. Tetrahedron 62, 7009–7013 (2006).
Basak, A., Chakrabarty, K., Ghosh, A. & Das, G. K. Theoretical study on the isomerization of propargyl derivative to conjugated diene under Au(i)-catalyzed reaction: a DFT study. Comput. Theor. Chem. 1083, 38–45 (2016).
Fu, J. et al. Gold-catalyzed rearrangement of allylic oxonium ylides: efficient synthesis of highly functionalized dihydrofuran-3-ones. Angew. Chem. Int. Ed. 52, 4198–4202 (2013).
Xiao, Y.-C. & Moberg, C. Silaborative carbocyclizations of 1,7-enynes. Diastereoselective preparation of chromane derivatives. Org. Lett. 18, 308–311 (2016).
Acknowledgements
L.Z. thanks NIH (grant no. R01GM123342) and NSF CHE (grant no. 1800525) for financial support, and NSF (grant no. MRI-1920299) for the acquisition of Bruker 500 MHz and 400 MHz NMR instruments. The DFT studies were performed using the computational facilities purchased with funds from the National Science Foundation (grant no. CNS-1725797) and administered by the Center for Scientific Computing (CSC). The CSC is supported by the California NanoSystems Institute and the Materials Research Science and Engineering Center (MRSEC; NSF grant no. DMR 1720256) at UC Santa Barbara.
Author information
Authors and Affiliations
Contributions
T.L. conducted the experiments and prepared a draft of the manuscript. X.C. synthesized the ligands and their gold catalysts and helped with the manuscript. P.Q. secured a postdoctoral fellowship from Wenzhou University for T.L. and participated in the chemistry design. L.Z. designed the chemistry and supervised its implementation and finalized the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Ryan Vander Linden and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures, DFT-optimized structure coordinates, X-ray diffraction data, chiral HPLC chromatographs and NMR spectra.
Supplementary Data 1
X-ray crystal data of compound 11.
Supplementary Data 2
X-ray crystal data of compound 6p.
Supplementary Data 3
X-ray crystal data of compound (S)-L3AuCl.
Rights and permissions
About this article
Cite this article
Li, T., Cheng, X., Qian, P. et al. Gold-catalysed asymmetric net addition of unactivated propargylic C–H bonds to tethered aldehydes. Nat Catal 4, 164–171 (2021). https://doi.org/10.1038/s41929-020-00569-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00569-8