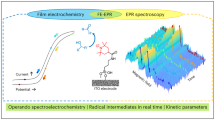
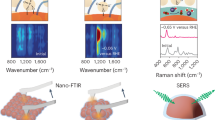
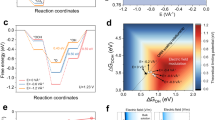
Abstract
Immobilized first-row transition metal complexes are potential low-cost electrocatalysts for selective CO2 conversion in the production of renewable fuels. Mechanistic understanding of their function is vital for the development of next-generation catalysts, although the poor surface sensitivity of many techniques makes this challenging. Here, a nickel bis(terpyridine) complex is introduced as a CO2 reduction electrocatalyst in a unique electrode geometry, sandwiched by thiol-anchoring moieties between two gold surfaces. Gap-plasmon-assisted surface-enhanced Raman scattering spectroscopy coupled with density functional theory calculations reveals that the nature of the anchoring group plays a pivotal role in the catalytic mechanism. Our in situ spectro-electrochemical measurement enables the detection of as few as eight molecules undergoing redox transformations in individual plasmonic hotspots, together with the calibration of electrical fields via vibrational Stark effects. This advance allows rapid exploration of non-resonant redox reactions at the few-molecule level and provides scope for future mechanistic studies of single molecules.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the University of Cambridge data repository at https://doi.org/10.17863/CAM.60379.
Code availability
The code for spectral matching using the earth mover algorithm is available from the University of Cambridge data repository at https://doi.org/10.17863/CAM.60379.
References
Baumberg, J. J., Aizpurua, J., Mikkelsen, M. H. & Smith, D. R. Extreme nanophotonics from ultrathin metallic gaps. Nat. Mater. 18, 668–678 (2019).
Pfisterer, J. H. K. & Domke, K. F. Unfolding the versatile potential of EC-TERS for electrocatalysis. Curr. Opin. Electrochem. 8, 96–102 (2018).
Kang, G., Yang, M., Mattei, M. S., Schatz, G. C. & Van Duyne, R. P. In situ nanoscale redox mapping using tip-enhanced Raman spectroscopy. Nano Lett. 19, 2106–2113 (2019).
Schmid, T., Opilik, L., Blum, C. & Zenobi, R. Nanoscale chemical imaging using tip-enhanced Raman spectroscopy: a critical review. Angew. Chem. Int. Ed. 52, 5940–5954 (2013).
Kumar, N. et al. Extending the plasmonic lifetime of tip-enhanced Raman spectroscopy probes. Phys. Chem. Chem. Phys. 18, 13710–13716 (2016).
Wu, D.-Y., Li, J.-F., Ren, B. & Tian, Z.-Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chem. Soc. Rev. 37, 1025–1041 (2008).
Zong, C., Chen, C. J., Zhang, M., Wu, D. Y. & Ren, B. Transient electrochemical surface-enhanced Raman spectroscopy: a millisecond time-resolved study of an electrochemical redox process. J. Am. Chem. Soc. 137, 11768–11774 (2015).
Wain, A. J. & O’Connell, M. A. Advances in surface-enhanced vibrational spectroscopy at electrochemical interfaces. Adv. Phys. X 2, 188–209 (2017).
Willets, K. A. & Van Duyne, R. P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 58, 267–297 (2007).
Benz, F. et al. SERS of individual nanoparticles on a mirror: size does matter, but so does shape. J. Phys. Chem. Lett. 7, 2264–2269 (2016).
Chazalviel, J.-N. & Allongue, P. On the origin of the efficient nanoparticle mediated electron transfer across a self-assembled monolayer. J. Am. Chem. Soc. 133, 762–764 (2011).
Zhao, J., Bradbury, C. R. & Fermín, D. J. Long-range electronic communication between metal nanoparticles and electrode surfaces separated by polyelectrolyte multilayer films. J. Phys. Chem. C 112, 6832–6841 (2008).
Shein, J. B., Lai, L. M. H., Eggers, P. K., Paddon-Row, M. N. & Gooding, J. J. Formation of efficient electron transfer pathways by adsorbing gold nanoparticles to self-assembled monolayer modified electrodes. Langmuir 25, 11121–11128 (2009).
Wang, L., Polyansky, D. E. & Concepcion, J. J. Self-assembled bilayers as an anchoring strategy: catalysts, chromophores, and chromophore-catalyst assemblies. J. Am. Chem. Soc. 141, 8020–8024 (2019).
Dalle, K. E. et al. Electro- and solar-driven fuel synthesis with first row transition metal complexes. Chem. Rev. 119, 2752–2875 (2019).
Materna, K. L., Crabtree, R. H. & Brudvig, G. W. Anchoring groups for photocatalytic water oxidation on metal oxide surfaces. Chem. Soc. Rev. 46, 6099–6110 (2017).
Clark, M. L. et al. CO2 reduction catalysts on gold electrode surfaces influenced by large electric fields. J. Am. Chem. Soc. 140, 17643–17655 (2018).
Cunillera, A. et al. Highly efficient Rh-catalysts immobilised by π-π stacking for the asymmetric hydroformylation of norbornene under continuous flow conditions. ChemCatChem 11, 2195–2205 (2019).
Kuehnel, M. F., Orchard, K. L., Dalle, K. E. & Reisner, E. Selective photocatalytic CO2 reduction in water through anchoring of a molecular Ni catalyst on CdS nanocrystals. J. Am. Chem. Soc. 139, 7217–7223 (2017).
Elgrishi, N., Chambers, M. B., Artero, V. & Fontecave, M. Terpyridine complexes of first row transition metals and electrochemical reduction of CO2 to CO. Phys. Chem. Chem. Phys. 16, 13635–13644 (2014).
Leung, J. J. et al. Solar-driven reduction of aqueous CO2 with a cobalt bis(terpyridine)-based photocathode. Nat. Catal. 2, 354–365 (2019).
Bae, J. H., Han, J.-H. & Chung, T. D. Electrochemistry at nanoporous interfaces: new opportunity for electrocatalysis. Phys. Chem. Chem. Phys. 14, 448–463 (2012).
Ai, L., Tian, T. & Jiang, J. Ultrathin graphene layers encapsulating nickel nanoparticles derived metal–organic frameworks for highly efficient electrocatalytic hydrogen and oxygen evolution reactions. ACS Sustain. Chem. Eng. 5, 4771–4777 (2017).
Yang, Y., Liu, Z. & Lian, T. Bulk transport and interfacial transfer dynamics of photogenerated carriers in CdSe quantum dot solid electrodes. Nano Lett. 13, 3678–3683 (2013).
Di Martino, G. et al. Tracking nanoelectrochemistry using individual plasmonic nanocavities. Nano Lett. 17, 4840–4845 (2017).
Jiang, S. et al. Investigation of cobalt phthalocyanine at the solid/liquid interface by electrochemical tip-enhanced Raman spectroscopy. J. Phys. Chem. C 123, 9852–9859 (2019).
Olalla, P. Optical transport and sensing in plexcitonic nanocavities. Opt. Express 21, 2649–2654 (2013).
Benz, F. et al. Generalized circuit model for coupled plasmonic systems. Opt. Express 23, 33255–33269 (2015).
De Nijs, B. et al. Unfolding the contents of sub-nm plasmonic gaps using normalising plasmon resonance spectroscopy. Faraday Discuss. 178, 185–193 (2015).
Gold nanoparticles. BBI Solutions https://bbisolutions.com/en/products/gold-reagents/gold-nanoparticles.html (2020).
Ge, A. et al. Interfacial structure and electric field probed by in situ electrochemical vibrational Stark effect spectroscopy and computational modeling. J. Phys. Chem. C 121, 18674–18682 (2017).
Boxer, S. G. Stark realities. J. Phys. Chem. B 113, 2972–2983 (2009).
Nelson, D. A. & Schultz, Z. D. Influence of optically rectified electric fields on the plasmonic photocatalysis of 4-nitrothiophenol and 4-aminothiophenol to 4,4-dimercaptoazobenzene. J. Phys. Chem. C 122, 8581–8588 (2018).
Compton, R. G. & Banks, C. E. Understanding Voltammetry (Imperial College Press, 2010).
Akkerman, H. B. et al. Electron tunneling through alkanedithiol self-assembled monolayers in large-area molecular junctions. Proc. Natl Acad. Sci. USA 104, 11161–11166 (2007).
Poon, J. et al. Altered electrochemistry at graphene- or alumina-modified electrodes: catalysis vs electrocatalysis in multistep electrode processes. J. Phys. Chem. C 119, 13777–13784 (2015).
Hu, Y. et al. Tracking mechanistic pathway of photocatalytic CO2 reaction at Ni sites using operando time-resolved spectroscopy. J. Am. Chem. Soc. 142, 5618–5626 (2020).
Farkas, E., Enyedy, É. A., Micera, G. & Garribba, E. Coordination modes of hydroxamic acids in copper(II), nickel(II) and zinc(II) mixed-ligand complexes in aqueous solution. Polyhedron 19, 1727–1736 (2000).
Readman, C. et al. Anomalously large spectral shifts near the quantum tunnelling limit in plasmonic rulers with subatomic resolution. Nano Lett. 19, 2051–2058 (2019).
Liu, Y. C., Hwang, B. J. & Jian, W. J. Effect of preparation conditions for roughening gold substrate by oxidation-reduction cycle on the surface-enhanced Raman spectroscopy of polypyrrole. Mater. Chem. Phys. 73, 129–134 (2002).
Carnegie, C. et al. Room-temperature optical picocavities below 1 nm3 accessing single-atom geometries. J. Phys. Chem. Lett. 9, 7146–7151 (2018).
Anderer, C., Näther, C. & Bensch, W. Bis(2,2’:6’,2”-terpyridine-K3 N,N’,N”)nickel(II) bis(perchlorate) hemihydrate. IUCrData 1, x161009 (2016); https://iucrdata.iucr.org/x/issues/2016/07/00/su4053/su4053.pdf
Becke, A. D. Density−functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Frisch, M. J. et al. Gaussian 09, Revision E (Gaussian, 2009).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Foster, J. P. & Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 102, 7211–7218 (1980).
Reed, A. E., Curtiss, L. A. & Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88, 899–926 (1988).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Tissandier, M. D. et al. The proton’s absolute aqueous enthalpy and Gibbs free energy of solvation from cluster-ion solvation data. J. Phys. Chem. A 102, 7787–7794 (1998).
Acknowledgements
We thank G. Di Martino for support with spectro-electrochemical cell design, B. de Nijs for support with Raman facilities and D.-B. Grys for support with understanding of polarized and unpolarized DFT. We acknowledge funding from the EPSRC (nos. EP/L027151/1 and EP/R013012/1) and Cambridge NanoDTC (no. EP/L015978/1 to D.W. and C.R.), and the ERC (no. 757850 BioNet to D.B. and T.F.). We are grateful to the UK Materials and Molecular Modelling Hub for computational resources, which is partially funded by EPSRC (no. EP/P020194/1). We acknowledge use of the research computing facility (Rosalind) at King’s College London (https://rosalind.kcl.ac.uk).
Author information
Authors and Affiliations
Contributions
D.W., Q.L., E. Reisner and J.J.B. conceived the research and developed the experiments. D.B., T.F. and E. Rosta carried out DFT calculations and provided input on catalytic interpretation. A.W. and E. Reisner provided input on interpretation of electrochemical and catalytic results. J.G. helped with spectral analysis. C.R. helped with synthesis of Ni(tpyS)2. D.W., Q.L., D.B., T.F. and J.J.B. analysed the data and wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Irina Chernyshova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16, Tables 1–5 and Notes 1–7.
Supplementary Data
Atomic coordinates of the optimized molecules.
Rights and permissions
About this article
Cite this article
Wright, D., Lin, Q., Berta, D. et al. Mechanistic study of an immobilized molecular electrocatalyst by in situ gap-plasmon-assisted spectro-electrochemistry. Nat Catal 4, 157–163 (2021). https://doi.org/10.1038/s41929-020-00566-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00566-x
This article is cited by
-
Plasmon-mediated chemical reactions
Nature Reviews Methods Primers (2023)
-
Capturing critical gem-diol intermediates and hydride transfer for anodic hydrogen production from 5-hydroxymethylfurfural
Nature Communications (2023)
-
Ultrafast charge transfer in mixed-dimensional WO3-x nanowire/WSe2 heterostructures for attomolar-level molecular sensing
Nature Communications (2023)
-
Photoluminescence upconversion in monolayer WSe2 activated by plasmonic cavities through resonant excitation of dark excitons
Nature Communications (2023)
-
Experimental characterization techniques for plasmon-assisted chemistry
Nature Reviews Chemistry (2022)