Abstract
Metal-exchanged zeolites have been widely used in industrial catalysis and separation, but fundamental understanding of their structure–property relationships has remained challenging, largely due to the lack of quantitative information concerning the atomic structures and reaction-relevant adsorption properties of the embedded metal active sites. Here, we report on using low-temperature reactive adsorption of NO to titrate copper-exchanged ZSM5 (Cu-ZSM5). Quantitative descriptors of the atomic structures and adsorption properties of Cu-ZSM5 are established by combining atomistic simulation, density functional theory c, operando molecular spectroscopy, chemisorption and titration measurements. These descriptors are then applied to interpret the catalytic performance of Cu-ZSM5 for NO decomposition. Linear correlations are established to bridge low-temperature adsorption analytics and high-temperature reaction kinetics, which are demonstrated to be generally applicable for understanding the structure–property relationships of metal-exchanged zeolites and foregrounded the development of advanced catalytic materials.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available on the Figshare platform at https://doi.org/10.6084/m9.figshare.13128506.v1 (ref. 55). Source data are provided with this paper.
References
Yang, J. C., Small, M. W., Grieshaber, R. V. & Nuzzo, R. G. Recent developments and applications of electron microscopy to heterogeneous catalysis. Chem. Soc. Rev. 41, 8179–8194 (2012).
Bordiga, S., Groppo, E., Agostini, G., van Bokhoven, J. A. & Lamberti, C. Reactivity of surface species in heterogeneous catalysts probed by in situ X-ray absorption techniques. Chem. Rev. 113, 1736–1850 (2013).
Zaera, F. New advances in the use of infrared absorption spectroscopy for the characterization of heterogeneous catalytic reactions. Chem. Soc. Rev. 43, 7624–7663 (2014).
Wachs, I. E. & Roberts, C. A. Monitoring surface metal oxide catalytic active sites with Raman spectroscopy. Chem. Soc. Rev. 39, 5002–5017 (2010).
Norskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Zhang, R. D., Liu, N., Lei, Z. G. & Chen, B. H. Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolite catalysts. Chem. Rev. 116, 3658–3721 (2016).
Lamberti, C. et al. XAFS, IR, and UV-vis study of the CuI environment in CuI-ZSM-5. J. Phys. Chem. B 101, 344–360 (1997).
Moden, B., Da Costa, P., Fonfe, B., Lee, D. K. & Iglesia, E. Kinetics and mechanism of steady-state catalytic NO decomposition reactions on Cu-ZSM5. J. Catal. 209, 75–86 (2002).
Snyder, B. E. R., Bols, M. L., Schoonheydt, R. A., Sels, B. F. & Solomon, E. I. Iron and copper active sites in zeolites and their correlation to metalloenzymes. Chem. Rev. 118, 2718–2768 (2018).
Giordanino, F. et al. Characterization of Cu-exchanged SSZ-13: a comparative FTIR, UV-Vis, and EPR study with Cu-ZSM-5 and Cu-β with similar Si/Al and Cu/Al ratios. Dalton Trans. 42, 12741–12761 (2013).
Woertink, J. S. et al. A [Cu2O]2+ core in Cu-ZSM-5, the active site in the oxidation of methane to methanol. Proc. Natl Acad. Sci. USA 106, 18908–18913 (2009).
Groothaert, M. H., Pierloot, K., Delabie, A. & Schoonheydt, R. A. Identification of Cu(ii) coordination structures in Cu-ZSM-5, based on a DFT/ab initio assignment of the EPR spectra. Phys. Chem. Chem. Phys. 5, 2135–2144 (2003).
Groothaert, M. H., van Bokhoven, J. A., Battiston, A. A., Weckhuysen, B. M. & Schoonheydt, R. A. Bis(μ-oxo)dicopper in Cu-ZSM-5 and its role in the decomposition of NO: a combined in situ XAFS, UV−vis−near-IR, and kinetic study. J. Am. Chem. Soc. 125, 7629–7640 (2003).
Sajith, P. K., Shiota, Y. & Yoshizawa, K. Role of acidic proton in the decomposition of NO over dimeric Cu(i) active sites in Cu-ZSM-5 catalyst: a QM/MM study. ACS Catal. 4, 2075–2085 (2014).
Moretti, G. et al. Dimeric Cu(i) species in Cu-ZSM-5 catalysts: the active sites for the NO decomposition. J. Catal. 232, 476–487 (2005).
Tsai, M. L. et al. [Cu2O]2+ active site formation in Cu-ZSM-5: geometric and electronic structure requirements for N2O activation. J. Am. Chem. Soc. 136, 3522–3529 (2014).
Da Costa, P., Moden, B., Meitzner, G. D., Lee, D. K. & Iglesia, E. Spectroscopic and chemical characterization of active and inactive Cu species in NO decomposition catalysts based on Cu-ZSM5. Phys. Chem. Chem. Phys. 4, 4590–4601 (2002).
Ravi, M. et al. Misconceptions and challenges in methane-to-methanol over transition-metal-exchanged zeolites. Nat. Catal. 2, 485–494 (2019).
Kustova, M. Y., Rasmussen, S. B., Kustov, A. L. & Christensen, C. H. Direct NO decomposition over conventional and mesoporous Cu-ZSM-5 and Cu-ZSM-11 catalysts: improved performance with hierarchical zeolites. Appl. Catal. B 67, 60–67 (2006).
Xie, P. F. et al. CoZSM-11 catalysts for N2O decomposition: effect of preparation methods and nature of active sites. Appl. Catal. B 170, 34–42 (2015).
Fanning, P. E. & Vannice, M. A. A DRIFTS study of Cu-ZSM-5 prior to and during its use for N2O decomposition. J. Catal. 207, 166–182 (2002).
Beutel, T., Sarkany, J., Lei, G. D., Yan, J. Y. & Sachtler, W. M. H. Redox chemistry of Cu/ZSM-5. J. Phys. Chem. 100, 845–851 (1996).
Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 39, 92–96 (1954); http://www.minsocam.org/ammin/AM39/AM39_92.pdf
Paolucci, C. et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 357, 898–903 (2017).
Latimer, A. A. et al. Understanding trends in C–H bond activation in heterogeneous catalysis. Nat. Mater. 16, 225–229 (2017).
Zhao, Z. J., Kulkarni, A., Vilella, L., Norskov, J. K. & Studt, F. Theoretical insights into the selective oxidation of methane to methanol in copper-exchanged mordenite. ACS Catal. 6, 3760–3766 (2016).
Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms.J. Phys. Condens. Matter 29, 273002 (2017).
Wang, S., He, Y., Jiao, W. Y., Wang, J. G. & Fan, W. B. Recent experimental and theoretical studies on Al siting/acid site distribution in zeolite framework. Curr. Opin. Chem. Eng. 23, 146–154 (2019).
Itadani, A. et al. New information related to the adsorption model of N2 on CuMFI at room temperature. J. Phys. Chem. C 111, 16701–16705 (2007).
Henriques, C. et al. An FT-IR study of NO adsorption over Cu-exchanged MFI catalysts: effect of Si/Al ratio, copper loading and catalyst pre-treatment. Appl. Catal. B 16, 79–95 (1998).
Kosinov, N., Liu, C., Hensen, E. J. M. & Pidko, E. A. Engineering of transition metal catalysts confined in zeolites. Chem. Mater. 30, 3177–3198 (2018).
Agarwal, N. et al. Aqueous Au–Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 358, 223–226 (2017).
Schneider, W. F., Hass, K. C., Ramprasad, R. & Adams, J. B. First-principles analysis of elementary steps in the catalytic decomposition of NO by Cu-exchanged zeolites. J. Phys. Chem. B 101, 4353–4357 (1997).
Fanson, P. T., Stradt, M. W., Lauterbach, J. & Delgass, W. N. The effect of Si/Al ratio and copper exchange level on isothermal kinetic rate oscillations for N2O decomposition over Cu-ZSM-5: a transient FTIR study. Appl. Catal. B 38, 331–347 (2002).
Hadjiivanov, K. I. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. 42, 71–144 (2000).
Morpurgo, S. A. DFT study on the mechanism of NO decomposition catalyzed by short-distance Cu(i) pairs in Cu-ZSM-5. Mol. Catal. 434, 96–105 (2017).
Izquierdo, R., Rodriguez, L. J., Anez, R. & Sierraalta, A. Direct catalytic decomposition of NO with Cu-ZSM-5: a DFT-ONIOM study. J. Mol. Catal. A Chem. 348, 55–62 (2011).
Konduru, M. V. & Chuang, S. S. C. Investigation of adsorbate reactivity during NO decomposition over different levels of copper ion-exchanged ZSM-5 using in situ IR technique. J. Phys. Chem. B 103, 5802–5813 (1999).
Kuroda, Y., Kumashiro, R., Yoshimoto, T. & Nagao, M. Characterization of active sites on copper ion-exchanged ZSM-5-type zeolite for NO decomposition reaction. Phys. Chem. Chem. Phys. 1, 649–656 (1999).
Lee, D. K. Thermodynamic features of the Cu-ZSM-5 catalyzed NO decomposition reaction. Korean J. Chem. Eng. 23, 547–554 (2006).
Aranovich, G. L. & Donohue, M. D. Phase loops in density-functional-theory calculations of adsorption in nanoscale pores. Phys. Rev. E 60, 5552–5560 (1999).
Aranovich, G. L. & Donohue, M. D. Adsorption compression: an important new aspect of adsorption behavior and capillarity. Langmuir 19, 2722–2735 (2003).
Smeets, P. J. et al. Direct NO and N2O decomposition and NO-assisted N2O decomposition over Cu-zeolites: elucidating the influence of the Cu–Cu distance on oxygen migration. J. Catal. 245, 358–368 (2007).
Teraishi, K. et al. Active site structure of Cu/ZSM-5: computational study. J. Phys. Chem. B 101, 8079–8085 (1997).
Sushkevich, V. L., Palagin, D., Ranocchiari, M. & van Bokhoven, J. A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523–527 (2017).
Verma, A. A. et al. NO oxidation: a probe reaction on Cu-SSZ-13. J. Catal. 312, 179–190 (2014).
Pappas, D. K. et al. The nuclearity of the active site for methane to methanol conversion in Cu-mordenite: a quantitative assessment. J. Am. Chem. Soc. 140, 15270–15278 (2018).
Narsimhan, K., Iyoki, K., Dinh, K. & Roman-Leshkov, Y. Catalytic oxidation of methane into methanol over copper-exchanged zeolites with oxygen at low temperature. ACS Cent. Sci. 2, 424–429 (2016).
Groothaert, M. H., Smeets, P. J., Sels, B. F., Jacobs, P. A. & Schoonheydt, R. A. Selective oxidation of methane by the bis(μ-oxo)dicopper core stabilized on ZSM-5 and mordenite zeolites. J. Am. Chem. Soc. 127, 1394–1395 (2005).
Kulkarni, A. R., Zhao, Z. J., Siahrostami, S., Norskov, J. K. & Studt, F. Cation-exchanged zeolites for the selective oxidation of methane to methanol. Catal. Sci. Technol. 8, 114–123 (2018).
Yumura, T., Hirose, Y., Wakasugi, T., Kuroda, Y. & Kobayashi, H. Roles of water molecules in modulating the reactivity of dioxygen-bound Cu-ZSM-5 toward methane: a theoretical prediction. ACS Catal. 6, 2487–2495 (2016).
Tomkins, P. et al. Isothermal cyclic conversion of methane into methanol over copper-exchanged zeolite at low temperature. Angew. Chem. Int. Ed. 55, 5467–5471 (2016).
Xie, P. F. et al. Catalytic decomposition of N2O over Cu-ZSM-11 catalysts. Microporous Mesoporous Mater. 191, 112–117 (2014).
Xing, B., Ma, J. H., Li, R. F. & Jiao, H. J. Location, distribution and acidity of Al substitution in ZSM-5 with different Si/Al ratios—a periodic DFT computation. Catal. Sci. Tech. 7, 5694–5708 (2017).
Xie, P. F. et al. Bridging adsorption analytics and catalytic kinetics for metal-exchanged zeolites. Figshare https://doi.org/10.6084/m9.figshare.13128506.v1 (2020).
Acknowledgements
This work was supported by the Department of Energy, Advanced Research Projects Agency-Energy (ARPA-E). P.X. and C.W. also acknowledge support from the Petroleum Research Fund, American Chemical Society. A.K. acknowledges the use of computing resources provided by the National Energy Research Scientific Computing Center (NERSC; a US Department of Energy Office of Science User Facility operated under contract number DE-AC02-05CH11231) and the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant number ACI-1548562.
Author information
Authors and Affiliations
Contributions
C.W. and P.X. conceived of the idea and experimental design. P.X. and T.P. carried out the experiments. P.X. and C.W. wrote the paper. M.D. and G.A. contributed to analysis of the NOad isotherms using Ono–Kondo coordinates. A.K. and J.G. performed DFT calculations for this work. All of the authors discussed the results and contributed to manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Dennis Palagin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Discussion, Tables 1–15, Figs. 1–38 and References.
Supplementary Data 1
Electronic structure calculations.
Source data
Source Data Fig. 1
Characterization of Cu sites in ZSM5.
Source Data Fig. 2
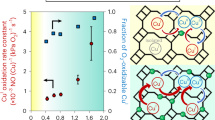
DFT-calculated fraction of Cu dimer in different ZSM5 frameworks.
Source Data Fig. 3
DFT-calculated pathways of reaction and adsorption of NO on Cu-ZSM5.
Source Data Fig. 4
Characterization and quantification of Cu dimers in Cu-ZSM5 zeolites.
Source Data Fig. 5
NO isothermal adsorption and corresponding analytics.
Source Data Fig. 6
Catalytic performance and kinetics of NO decomposition.
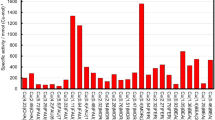
Source Data Fig. 7
Catalytic study of MTM using Cu-ZSM5 from the literature and this work.
Rights and permissions
About this article
Cite this article
Xie, P., Pu, T., Aranovich, G. et al. Bridging adsorption analytics and catalytic kinetics for metal-exchanged zeolites. Nat Catal 4, 144–156 (2021). https://doi.org/10.1038/s41929-020-00555-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00555-0