Abstract
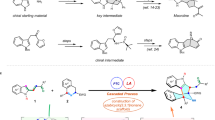
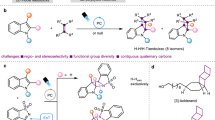
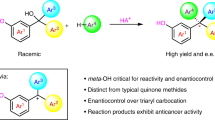
While synthetic chemistry has experienced substantial development in the past century, challenges still remain to fully satisfy the needs in drug development. A bias in sampling linear and disc-shaped molecules in drug discovery over spherical ones has existed due to the lack of efficient access to the latter chemical space. Specifically, efficient strategies to synthesize tetraarylmethanes, a unique family of spherical molecules, has remained scarce. In particular, there has been essentially no efficient asymmetric synthesis of chiral tetraarylmethanes due to the overwhelming steric congestion and challenging stereocontrol encountered in assembly of the all-aryl-substituted quaternary stereocentre. Here we disclose an efficient catalytic synthesis of chiral tetraarylmethanes with high enantioselectivity via a stereoconvergent formal nucleophilic substitution reaction. Control experiments and density functional theory calculations provided strong support on hydrogen bonding interactions as the key elements to successful stereocontrol. The obtained enantioenriched products showed impressive preliminary anticancer activities.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated and analysed during this study are included in this Article and its Supplementary Information. They are also available from the authors upon reasonable request. The X-ray crystallographic coordinates for the structures of 3ae′ (derivative of 3ae) and 5a have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 1935077 and CCDC 1935078, respectively, and can be obtained free of charge from the CCDC via http://www.ccdc.cam.ac.uk/data_request/cif.
References
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science 363, eaat0805 (2019).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Franklin, M. R. Induction of rat liver drug-metabolizing enzymes by heterocycle-containing mono-, di-, tri- and tetra-arylmethanes. Biochem. Pharmacol. 46, 683–689 (1993).
El-Kaderi, H. M. et al. Designed synthesis of 3D covalent organic frameworks. Science 316, 268–272 (2007).
Ganesan, P. et al. Tetrahedral n-type materials: efficient quenching of the excitation of p-type polymers in amorphous films. J. Am. Chem. Soc. 127, 14530–14531 (2005).
Bonardi, F. et al. Probing the SecYEG translocation pore size with preproteins conjugated with sizable rigid spherical molecules. Proc. Natl Acad. Sci. USA 108, 7775–7780 (2011).
Huang, X. et al. Self-assembly of morphology-tunable architectures from tetraarylmethane derivatives for targeted drug delivery. Langmuir 29, 3223–3233 (2013).
Dong, J., Liu, Y. & Cui, Y. Chiral porous organic frameworks for asymmetric heterogeneous catalysis and gas chromatographic separation. Chem. Commun. 50, 14949–14952 (2014).
Wu, C. et al. Highly conjugated three-dimensional covalent organic frameworks based on spirobifluorene for perovskite solar cell enhancement. J. Am. Chem. Soc. 140, 10016–10024 (2018).
Mercado, R. et al. In silico design of 2D and 3D covalent organic frameworks for methane storage applications. Chem. Mater. 30, 5069–5086 (2018).
Oniki, J., Moriuchi, T., Kamochi, K., Tobisu, M. & Amaya, T. Linear [3]spirobifluorenylene: an S-shaped molecular geometry of p-oligophenyls. J. Am. Chem. Soc. 141, 18238–18245 (2019).
Schoepfle, C. S. & Trepp, S. G. The reaction between triarylmethyl halides and phenylmagnesium bromide. II. J. Am. Chem. Soc. 58, 791–794 (1936).
Adams, R., Hine, J. & Campbell, J. Triarylpyridylmethanes. J. Am. Chem. Soc. 71, 387–390 (1949).
Nambo, M., Yar, M., Smith, J. D. & Crudden, C. M. The concise synthesis of unsymmetric triarylacetonitriles via Pd-catalyzed sequential arylation: a new synthetic approach to tri- and tetraarylmethanes. Org. Lett. 17, 50–53 (2015).
Nambo, M., Yim, J. C.-H., Gowler, K. G. & Crudden, C. M. Synthesis of tetraarylmethanes by the triflic acid-promoted formal cross-dehydrogenative coupling of triarylmethanes with arenes. Synlett 28, 2936–2940 (2017).
Zhang, S., Kim, B.-S., Wu, C., Mao, J. & Walsh, P. J. Palladium-catalysed synthesis of triaryl(heteroaryl)methanes. Nat. Commun. 8, 14641 (2017).
Griffin, P. J., Fava, M. A., Whittaker, J. T., Kolonko, K. J. & Catino, A. J. Synthesis of tetraarylmethanes via a Friedel-Crafts cyclization/desulfurization strategy. Tetrahedron Lett. 59, 3999–4002 (2018).
Roy, D. & Panda, G. A dehydrative arylation and thiolation of tertiary alcohols catalyzed by in situ generated triflic acid - viable protocol for C–C and C–S bond formation. Tetrahedron 74, 6270–6277 (2018).
Palchaudhuri, R., Nesterenko, V. & Hergenrother, P. J. The complex role of the triphenylmethyl motif in anticancer compounds. J. Am. Chem. Soc. 130, 10274–10281 (2018).
Kshatriya, R., Jejurkar, V. P. & Saha, S. Advances in the catalytic synthesis of triarylmethanes (TRAMs). Eur. J. Org. Chem. 2019, 3818–3841 (2019).
Matthew, S. C., Glasspoole, B. W., Eisenberger, P. & Crudden, C. M. Synthesis of enantiomerically enriched triarylmethanes by enantiospecific Suzuki–Miyaura cross-coupling reactions. J. Am. Chem. Soc. 136, 5828–5831 (2014).
Huang, Y. & Hayashi, T. Asymmetric synthesis of triarylmethanes by rhodium-catalyzed enantioselective arylation of diarylmethylamines with arylboroxines. J. Am. Chem. Soc. 137, 7556–7559 (2015).
Pan, T. et al. CuH-catalyzed asymmetric 1,6-conjugate reduction of p-quinone methides: enantioselective synthesis of triarylmethanes and 1,1,2-triarylethanes. Org. Lett. 21, 6397–6402 (2019).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014).
Prakash, J. & Marek, I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem. Commun. 47, 4593–4623 (2011).
Tsuchida, K., Senda, Y., Nakajima, K. & Nishibayashi, Y. Construction of chiral tri- and tetra-arylmethanes bearing quaternary carbon centers: copper-catalyzed enantioselective propargylation of indoles with propargylic esters. Angew. Chem. Int. Ed. 55, 9728–9732 (2016).
Mahlau, M. & List, B. Asymmetric counteranion-directed catalysis: concept, definition, and applications. Angew. Chem. Int. Ed. 52, 518–533 (2013).
Brak, K. & Jacobsen, E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Ed. 52, 534–561 (2013).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Doyle, A. G. & Jacobsen, E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
Wang, Z. & Sun, J. Recent advances in catalytic asymmetric reactions of o-quinone methides. Synthesis 47, 3629–3644 (2015).
Caruana, L., Fochi, M. & Bernardi, L. The emergence of quinone methides in asymmetric organocatalysis. Molecules 20, 11733–11764 (2019).
Li, W., Xu, X., Zhang, P. & Li, P. Recent advances in the catalytic enantioselective reactions of para-quinone methides. Chem. Asian J. 13, 2350–2359 (2018).
El-Sepelgy, O., Haseloff, S., Alamsetti, S. K. & Schneider, C. Brønsted acid catalyzed, conjugate addition of β-dicarbonyls to in situ generated ortho-quinone methides—enantioselective synthesis of 4-aryl-4H-chromenes. Angew. Chem. Int. Ed. 53, 7923–7927 (2014).
Wang, Z. et al. Organocatalytic asymmetric synthesis of 1,1-diarylethanes by transfer hydrogenation. J. Am. Chem. Soc. 137, 383–389 (2015).
Wang, Z., Wong, Y. F. & Sun, J. Catalytic asymmetric 1,6-conjugate addition of para-quinone methides: formation of all-carbon quaternary stereocenters. Angew. Chem. Int. Ed. 54, 13711–13714 (2015).
Zhao, J.-J., Sun, S.-B., He, S.-H., Wu, Q. & Shi, F. Catalytic asymmetric inverse-electron-demand oxa-Diels–Alder reaction of in situ generated ortho-quinone methides with 3-methyl-2-vinylindoles. Angew. Chem. Int. Ed. 54, 5460–5464 (2015).
Xie, Y. & List, B. Catalytic asymmetric intramolecular [4+2] cycloaddition of in situ generated ortho-quinone methides. Angew. Chem. Int. Ed. 56, 4936–4940 (2017).
Lin, J.-S. et al. Cu/chiral phosphoric acid-catalyzed asymmetric three-component radical-initiated 1,2-dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 141, 1074–1083 (2019).
Ma, D., Miao, C.-B. & Sun, J. Catalytic enantioselective House–Meinwald rearrangement: efficient construction of all-carbon quaternary stereocenters. J. Am. Chem. Soc. 141, 13783–13787 (2019).
Zhang, H.-H. et al. Design and enantioselective construction of axially chiral naphthyl-indole skeletons. Angew. Chem. Int. Ed. 56, 116–121 (2017).
Sundberg, R. J. Indoles (Academic Press, 1996).
Kochanowska-Karamyan, A. J. & Hamann, M. T. Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem. Rev. 110, 4489–4497 (2010).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1568 (2004).
Uraguchi, D. & Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004).
Frisch, M. J. et al. Gaussian 16, Revision A.03 (Gaussian Inc., 2016).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Zhao, Y. & Truhlar, D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167 (2008).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Acknowledgements
We thank the Research Grants Council of Hong Kong (16302617, 16302318), National Natural Science Foundation of China (91956114, 21877092), Shenzhen Science and Technology Innovation Committee (JCYJ20170818113708560, JCYJ20160229205441091, JCYJ20200109141408054) and the National Science Foundation (CHE-1764328 to K.N.H) for financial support of this work. Calculations were performed on the Hoffman2 cluster at UCLA and the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation (OCI-1053575). We also thank H. H. Y. Sung for help with structure elucidation.
Author information
Authors and Affiliations
Contributions
X.L. conceived the project, performed the experiments and wrote the paper. M.D. and Q.S. performed DFT calculations. K.N.H. directed the DFT calculations and mechanism analysis. Z.D. performed the cytotoxicity experiments. G.Z. directed the cytotoxicity study. J.S. conceived and directed the project and wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.S. and X.L. are inventors on US patent application no. 62/918,404 submitted by the Hong Kong University of Science and Technology, which covers the catalytic system and its application in synthetic transformations. Other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Fig.1, Tables 1–29, NMR spectra and HPLC traces.
Supplementary Data 1
Crystallographic data for compound 3ae′.
Supplementary Data 2
Crystallographic data for compound 5a.
Supplementary Data 3
The atomic coordinates of DFT-computed structures.
Rights and permissions
About this article
Cite this article
Li, X., Duan, M., Deng, Z. et al. Catalytic enantioselective synthesis of chiral tetraarylmethanes. Nat Catal 3, 1010–1019 (2020). https://doi.org/10.1038/s41929-020-00535-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00535-4
This article is cited by
-
Enantioselective synthesis of tetraarylmethanes through meta-hydroxyl-directed benzylic substitution
Nature Synthesis (2023)
-
Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes
Nature Chemistry (2022)
-
Quinone methides and indole imine methides as intermediates in enantioselective catalysis
Nature Synthesis (2022)
-
Organocatalytic enantioselective dearomatization of thiophenes by 1,10-conjugate addition of indole imine methides
Nature Communications (2021)
-
Redox-enabled direct stereoconvergent heteroarylation of simple alcohols
Nature Communications (2021)