Abstract
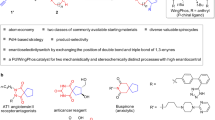
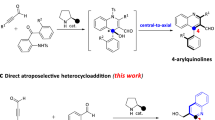
Asymmetric domino electrophilic halocyclizations are highly useful in the synthesis of structurally complex and pharmaceutically important compounds. Although some studies aimed at catalytic and enantioselective polyene cyclizations are documented, the chiral products have been limited to fused rings. Here, we report an efficient and highly enantio- and diastereoselective halocyclization and spiroketalization of olefinic keto-acids. Instead of electron-deficient thiourea, in this study electron-rich thiourea catalysts are crucial for high enantioselectivity. The resulting spiro compounds are privileged cores of many drugs and natural products. Our experimental and computational studies revealed that the reaction proceeded via a double dynamic–kinetic resolution mechanism. We anticipate that this work will stimulate the synthesis of other multifunctional compounds via electrophilic halocyclization.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The findings of this study are available within the paper and its Supplementary Information. Crystallographic parameters for compounds 3a, 4a-Br, 5 and 6 are available free of charge from the Cambridge Crystallographic Data Centre under CCDC 1978126 (3a), CCDC 1978123 (4a-Br), CCDC 1978125 (5) and 1978124 (6). All data are available from the authors upon reasonable request.
References
Rodríguez, F. & Fañanás, F. J. in Handbook of Cyclization Reactions Vol. 2 (ed. Ma, S.) 951−990 (Wiley-VCH, 2010).
Ranganathan, S., Muraleedharan, K. M., Vaish, N. K. & Jayaraman, N. Halo- and selenolactonisation: the two major strategies for cyclofunctionalisation. Tetrahedron 60, 5273–5308 (2004).
Chemler, S. R. & Bovino, M. T. Catalytic aminohalogenation of alkenes and alkynes. ACS Catal. 3, 1076–1091 (2013).
Neverov, A. A. & Brown, R. S. Br+ and I+ transfer from the halonium Ions of adamantylideneadamantane to acceptor olefins. Halocyclization of 1,ω-alkenols and alkenoic acids proceeds via reversibly formed intermediates. J. Org. Chem. 61, 962–968 (1996).
Denmark, S. E., Burk, M. T. & Hoover, A. J. On the absolute configurational stability of bromonium and chloronium ions. J. Am. Chem. Soc. 132, 1232–1233 (2010).
Chen, G. & Ma, S. Enantioselective halocyclization reactions for the synthesis of chiral cyclic compounds. Angew. Chem. Int. Ed. 49, 8306–8308 (2010).
Castellanos, A. & Fletcher, S. P. Current methods for asymmetric halogenation of olefins. Chem. Eur. J. 17, 5766–5776 (2011).
Snyder, S. A., Treitler, D. S. & Brucks, A. P. Halonium-induced cyclization reactions. Aldrichimica Acta 44, 27 (2011).
Hennecke, U. New catalytic approaches towards the enantioselective halogenation of alkenes. Chem. Asian J. 7, 456–465 (2012).
Denmark, S. E., Kuester, W. E. & Burk, M. T. Catalytic, asymmetric halofunctionalization of alkenes—a critical perspective. Angew. Chem. Int. Ed. 51, 10938–10935 (2012).
Cheng, Y. A., Yu, W. Z. & Yeung, Y.-Y. Recent advances in asymmetric intra- and intermolecular halofunctionalizations of alkenes. Org. Biomol. Chem. 12, 2333–2343 (2014).
Cochrane, N. A., Nguyen, H. & Gagne, M. R. Catalytic enantioselective cyclization and C3-fluorination of polyenes. J. Am. Chem. Soc. 135, 628–631 (2013).
Snyder, S. A., Treitler, D. S. & Schall, A. A two-step mimic for direct, asymmetric bromonium- and chloronium-induced polyene cyclizations. Tetrahedron 66, 4796–4804 (2010).
Grayfer, T. D., Retailleau, P., Dodd, R. H., Dubois, J. & Cariou, K. Chemodivergent, tunable, and selective iodine(iii)-mediated bromo-functionalizations of polyprenoids. Org. Lett. 19, 4766–4769 (2017).
Sawamura, Y., Nakatsuji, H., Sakakura, A. & Ishihara, K. “Phosphite–urea” cooperative high-turnover catalysts for the highly selective bromocyclization of homogeranylarenes. Chem. Sci. 4, 4181–4186 (2013).
Snyder, S. A., Treitler, D. S. & Brucks, A. P. Simple reagents for direct halonium-induced polyene cyclizations. J. Am. Chem. Soc. 132, 14303–14314 (2010).
Snyder, S. A. & Treitler, D. S. Et2SBr·SbCl5Br: an effective reagent for direct bromonium-induced polyene cyclizations. Angew. Chem. Int. Ed. 48, 7899–7903 (2009).
Arnold, A. M., Pöthig, A., Drees, M. & Gulder, T. NXS, morpholine, and HFIP: the ideal combination for biomimetic haliranium-induced polyene cyclizations. J. Am. Chem. Soc. 140, 4344–4353 (2018).
Sakakura, A., Ukai, A. & Ishihara, K. Enantioselective halocyclization of polyprenoids induced by nucleophilic phosphoramidites. Nature 445, 900–903 (2007).
Sawamura, Y., Ogura, Y., Nakatsuji, H., Sakakura, A. & Ishihara, K. Enantioselective bromocyclization of 2-geranylphenols induced by chiral phosphite–urea bifunctional catalysts. Chem. Commun. 52, 6068–6071 (2016).
Samanta, R. C. & Yamamoto, H. Catalytic asymmetric bromocyclization of polyenes. J. Am. Chem. Soc. 139, 1460–1463 (2017).
Kotke, M. & Schreiner, P. R. Acid-free, organocatalytic acetalization. Tetrahedron 62, 434–439 (2006).
Yousefi, R. et al. Catalytic, enantioselective synthesis of cyclic carbamates from dialkyl amines by CO2-capture: discovery, development, and mechanism. J. Am. Chem. Soc. 141, 618–625 (2019).
Vara, B. A., Struble, T. J., Wang, W., Dobish, M. C. & Johnston, J. N. Enantioselective small molecule synthesis by carbon dioxide fixation using a dual Brønsted acid/base organocatalyst. J. Am. Chem. Soc. 137, 7302–7305 (2015).
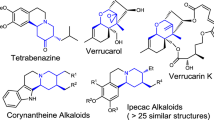
Zhang, F., Zhang, S. & Tu, Y. Recent progress in the isolation, bioactivity, biosynthesis, and total synthesis of natural spiroketals. Nat. Prod. Rep. 35, 75 (2018).
Aho, J. E., Pihko, P. M. & Rissa, T. K. Nonanomeric spiroketals in natural products: structures, sources, and synthetic strategies. Chem. Rev. 105, 4406–4440 (2005).
Galloway, W. R. J. D., Isidro-Llobet, A. & Spring, D. R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 1, 80 (2010).
Singh, G. S. & Desta, Z. Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 112, 6104–6155 (2012).
Painter, T. O. et al. Skeletal diversification via heteroatom linkage control: preparation of bicyclic and spirocyclic scaffolds from N-substituted homopropargyl alcohols. J. Org. Chem. 78, 3720–3730 (2013).
Guérot, C., Tchitchanov, B. H., Knust, H. & Carreira, E. M. Synthesis of novel angular spirocyclic azetidines. Org. Lett. 13, 780–783 (2011).
Burkhard, J. A. et al. Synthesis of azaspirocycles and their evaluation in drug discovery. Angew. Chem. Int. Ed. 49, 3524–3527 (2010).
Burkhard, J. A., Guérot, C., Knust, H., Rogers-Evans, M. & Carreira, E. M. Synthesis and structural analysis of a new class of azaspiro[3.3]heptanes as building blocks for medicinal chemistry. Org. Lett. 12, 1944–1947 (2010).
Wuitschik, G. et al. Spirocyclic oxetanes: synthesis and properties. Angew. Chem. Int. Ed. 47, 4512–4515 (2008).
Čorić, I. & List, B. Asymmetric spiroacetalization catalysed by confined Brønsted acids. Nature 483, 315–319 (2012).
Zinzalla, G., Milroy, L.-G. & Ley, S. V. Chemical variation of natural product-like scaffolds: design and synthesis of spiroketal derivatives. Org. Biomol. Chem. 4, 1977–2002 (2006).
Kalyani, D., Kornfilt, D. J.-P., Burk, M. T. & Denmark, S. E. in Lewis Base Catalysis in Organic Synthesis (eds Vedejs, E. & Denmark, S. E.) Ch. 24 (Wiley-VCH, 2016).
Zhou, L., Tan, C. K., Jiang, X. & Yeung, Y.-Y. Asymmetric bromolactonization using amino-thiocarbamate catalyst. J. Am. Chem. Soc. 132, 15474–15476 (2010).
Schreiner, P. R. & Wittkopp, A. H-bonding additives act like Lewis acid catalysts. Org. Lett. 4, 217–220 (2002).
Banik, S. M., Levina, A., Hyde, A. M. & Jacobsen, E. N. Lewis acid enhancement by hydrogen-bond donors for asymmetric catalysis. Science 358, 761–764 (2017).
Doyle, A. G. & Jacobsen, E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
Takemoto, Y. Recognition and activation by ureas and thioureas: stereoselective reactions using ureas and thioureas as hydrogen-bonding donors. Org. Biomol. Chem. 3, 4299–4306 (2005).
Zhang, D.-W. et al. Two new diterpenoids from cell cultures of Salvia miltiorrhiza. Chem. Pharm. Bull. 61, 576–580 (2013).
Ashtekar, K. D. et al. A new tool to guide halofunctionalization reactions: the halenium affinity (HalA) scale. J. Am. Chem. Soc. 136, 13355–13362 (2014).
Park, Y., Schindler, C. S. & Jacobsen, E. N. Enantioselective aza-Sakurai cyclizations: dual role of thiourea as H‑bond donor and Lewis base. J. Am. Chem. Soc. 138, 14848–14851 (2016).
Boyle, P. D. & Godfrey, S. M. The reactions of sulfur and selenium donor molecules with dihalogens and interhalogens. Coord. Chem. Rev. 223, 265–299 (2001).
Jakab, G., Hosseini, A., Hausmann, H. & Schreiner, P. R. Mild and selective organocatalytic iodination of activated aromatic compounds. Synthesis 45, 1635–1640 (2013).
Piers, E., Britton, R. W., Beraghty, M. B., Keziere, R. J. & Smillie, R. D. Stereoselective total synthesis of copa and ylango sesquiterpenoids: preparation of (–)-(1S,4S,5R,7R)-1,7-dimethyl-4-isopropylbicyclo[3.2.1.]octa-6,8-dione and (+)-(1R,4S,5S,7S)-1,7-dimethyl-4-isopropylbicyclo[3.2.1]octa-6,8-dione. Can. J. Chem. 53, 2827–2837 (1975).
Newberry, R. W. & Raines, R. T. The n→π* interaction. Acc. Chem. Res. 50, 1838–1846 (2017).
Sahariah, B. & Sarma, B. K. Relative orientation of the carbonyls groups determines the nature of orbital interactions in carbonyl–carbonyl short contacts. Chem. Sci. 10, 909–917 (2019).
Glendening, E. D. et al. NBO 7.0 (University of Wisconsin, 2018); https://nbo7.chem.wisc.edu/biblio_css.htm
Becke, A. The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design (John Wiley, 2007).
Frisch, M. J. et al. Gaussian 09 Revision D.01 (Gaussian Inc., 2009).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Neese, F. Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 8, e1327 (2018).
Kozuch, S. & Martin, J. M. Spin‐component‐scaled double hybrids: an extensive search for the best fifth‐rung functionals blending DFT and perturbation theory. J. Comput. Chem. 34, 2327–2344 (2013).
Acknowledgements
This work was supported by Hong Kong Special Administrative Region General Research Funding (grant number CUHK14304918), the Chinese University of Hong Kong Direct Grant (grant number 4053329) and Innovation and Technology Commission to the State Key Laboratory of Synthetic Chemistry (GHP/004/16GD). We thank Professor B. List (Max-Planck-Institut für Kohlenforschung) for a helpful discussion.
Author information
Authors and Affiliations
Contributions
Y.-Y.Y. conceived of and directed the project. T.Z. performed the experimental works. W.-H.N. assisted the preparation of some starting materials. Y.-L.S.T. directed the DFT calculations and mechanism analysis. X.W. performed the DFT calculations. T.Z., X.W., Y.-L.S.T. and Y.-Y.Y. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16, Tables 1–4, Methods, References
Supplementary Data
Supplementary Data for DFT
Supplementary Data 1
CIF file for the crystallographic data of compound 3a.
Supplementary Data 2
CIF file for the crystallographic data of compound 4a-Br.
Supplementary Data 3
CIF file for the crystallographic data of compound 5.
Supplementary Data 4
CIF file for the crystallographic data of compound 6.
Rights and permissions
About this article
Cite this article
Zheng, T., Wang, X., Ng, WH. et al. Catalytic enantio- and diastereoselective domino halocyclization and spiroketalization. Nat Catal 3, 993–1001 (2020). https://doi.org/10.1038/s41929-020-00530-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00530-9
This article is cited by
-
Iodomethane as an organocatalyst for the aerobic ortho-selective trifluoromethylation of pyridines
Science China Chemistry (2023)