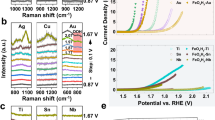
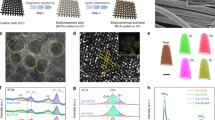
Abstract
Multimetal oxyhydroxides have recently been reported that outperform noble metal catalysts for oxygen evolution reaction (OER). In such 3d-metal-based catalysts, the oxidation cycle of 3d metals has been posited to act as the OER thermodynamic-limiting process; however, further tuning of its energetics is challenging due to similarities among the electronic structures of neighbouring 3d metal modulators. Here we report a strategy to reprogram the Fe, Co and Ni oxidation cycles by incorporating high-valence transition-metal modulators X (X = W, Mo, Nb, Ta, Re and MoW). We use in situ and ex situ soft and hard X-ray absorption spectroscopies to characterize the oxidation transition in modulated NiFeX and FeCoX oxyhydroxide catalysts, and conclude that the lower OER overpotential is facilitated by the readier oxidation transition of 3d metals enabled by high-valence modulators. We report an ~17-fold mass activity enhancement compared with that for the OER catalysts widely employed in industrial water-splitting electrolysers.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available on the Zenodo platform (https://zenodo.org/record/4008830) (ref. 31).
References
Zhang, J., Zhao, Z., Xia, Z. & Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nano. 10, 444–452 (2015).
Ng, J. W. D. et al. Gold-supported cerium-doped NiOx catalysts for water oxidation. Nat. Energy 1, 16053 (2016).
Liang, Y. et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 10, 780–786 (2011).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 355, eaad4998 (2017).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014 (2016).
Bergmann, A. et al. Unified structural motifs of the catalytically active state of Co(oxyhydr)oxides during the electrochemical oxygen evolution reaction. Nat. Catal. 1, 711–719 (2018).
Guan, J. et al. Water oxidation on a mononuclear manganese heterogeneous catalyst. Nat. Catal. 1, 870–877 (2018).
Martin-Sabi, M. et al. Redox tuning the Weakley-type polyoxometalate archetype for the oxygen evolution reaction. Nat. Catal. 1, 208–213 (2018).
Roy, C. et al. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 1, 820–829 (2018).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Jouny, M., Luc, W. & Jiao, F. General Techno-Economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Galán-Mascarós, J. R. Water oxidation at electrodes modified with earth-abundant transition-metal catalysts. ChemElectroChem 2, 37–50 (2015).
Subbaraman, R. et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012).
Roger, I., Shipman, M. A. & Symes, M. D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 1, 0003 (2017).
Fabbri, E. et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 16, 925–931 (2017).
Zhang, B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352, 333–337 (2016).
Rosalbino, F., Delsante, S., Borzone, G. & Scavino, G. Electrocatalytic activity of crystalline Ni–Co–M (M = Cr, Mn, Cu) alloys on the oxygen evolution reaction in an alkaline environment. Int. J. Hydrog. Energy 38, 10170–10177 (2013).
Chen, J. Y. C., Miller, J. T., Gerken, J. B. & Stahl, S. S. Inverse spinel NiFeAlO4 as a highly active oxygen evolution electrocatalyst: promotion of activity by a redox-inert metal ion. Energy Environ. Sci. 7, 1382–1386 (2014).
Gerken, J. B., Shaner, S. E., Masse, R. C., Porubsky, N. J. & Stahl, S. S. A survey of diverse earth abundant oxygen evolution electrocatalysts showing enhanced activity from Ni-Fe oxides containing a third metal. Energy Environ. Sci. 7, 2376–2382 (2014).
Friebel, D. et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Bajdich, M., García-Mota, M., Vojvodic, A., Nørskov, J. K. & Bell, A. T. Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc. 135, 13521–13530 (2013).
Novák, M. et al. Primary oxide minerals in the system WO3–Nb2O5–TiO2–Fe2O3–FeO and their breakdown products from the pegmatite No. 3 at Dolní Bory-Hatě, Czech Republic. Eur. J. Mineral. 20, 487–499 (2008).
Kuepper, K. et al. Electronic and magnetic properties of highly ordered Sr2FeMoO6. Phys. Stat. Sol. (a) 201, 3252–3256 (2004).
Liu, X., Yang, W. & Liu, Z. Recent progress on synchrotron-based in-situ soft X-ray spectroscopy for energy materials. Adv. Mater. 26, 7710–7729 (2014).
de Groot, F. M. F. et al. 1s2p resonant inelastic X-ray scattering of iron oxides. J. Phys. Chem. B 109, 20751–20762 (2005).
Mitsui, T. in Magmas Under Pressure (eds Kono, Y. & Sanloup, C.) 179–210 (Elsevier, 2018).
Zheng, X. et al. Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat. Chem. 10, 149–154 (2018).
Liu, P. F., Yang, S., Zheng, L. R., Zhang, B. & Yang, H. G. Mo6+ activated multimetal oxygen-evolving catalysts. Chem. Sci. 8, 3484–3488 (2017).
Liu, P. F., Yang, S., Zheng, L. R., Zhang, B. & Yang, H. G. Electrochemical etching of α-cobalt hydroxide for improvement of oxygen evolution reaction. J. Mater. Chem. A 4, 9578–9584 (2016).
Qiu, Z., Tai, C.-W., Niklasson, G. A. & Edvinsson, T. Direct observation of active catalyst surface phases and the effect of dynamic self-optimization in NiFe-layered double hydroxides for alkaline water splitting. Energy Environ. Sci. 12, 572–581 (2019).
Zhang, B. et al. High-valence metals improve OER performance by modulating 3d metal oxidation cycle energetics. Zenodo Digital Repository https://doi.org/10.5281/zenodo.4008830 (2020).
Acknowledgements
This work was supported by MOST (grant no. 2016YFA0203302), NSFC (grant nos. 21875042, 21634003 and 51573027), STCSM (grant nos. 16JC1400702 and 18QA1400800), SHMEC (grant no. 2017-01-07-00-07-E00062) and Yanchang Petroleum Group. This work was also supported by The Programme for Professor of Eastern Scholar at Shanghai Institutions of Higher Learning. This work was supported by the Ontario Research Fund—Research Excellence Program, NSERC and the CIFAR Bio-Inspired Solar Energy program. This work has also benefited from the use of the SGM beamlines at Canadian Light Source; the 1W1B and 4B9B beamlines at the Beijing Synchrotron Radiation Facility; the BL14W1, BL08U1-A beamline at Shanghai Synchrotron Radiation Facility; and the 44A beamline at Taiwan Photon Source (TPS). Mössbauer spectroscopy measurements were conducted at the Advanced Photon Source, a Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357. We acknowledge the Paul Scherrer Institut, Villigen, Switzerland, for provision of synchrotron radiation beamtime at the beamline SuperXAS of the SLS and would like to thank M. Nachtegaal for assistance. We thank M. García-Melchor and Y. Zhang for discussions on DFT calculations. We thank J. Wu for the assistance with the TEM measurements. We thank R. Wolowiec and D. Kopilovic for their assistance. For computer time, this research used the resources of the Supercomputing Laboratory at KAUST.
Author information
Authors and Affiliations
Contributions
E.H.S., H.P., B.Z. and L.C. supervised the project. B.Z. designed the project. L.W. and B.Z. carried out the experiments. Z.C., S.M.K. and Z.W. carried out the DFT simulations. L.W., X.Z., L. Zhang, Y.W., C.W.P., L. Zheng and J.L. carried out XAS measurements. T.R. assisted in situ XAS experiments. L.W., F.P.G.A., R.C. and J.L. performed the XAS results analysis. O.V., Z.W. and P.D.L. assisted with the DFT simulations. W.B. and E.E.A. carried out the Mössbauer spectroscopy experiment and data analysis. C.T.D. and Y.H. contributed to discussions about the experiments. Y.J. and Y.L. contributed to the discussions about DFT simulations. Y.Z. assisted with TEM and XRD measurements. B.Z., L.W., Z.C., F.P.G.A., S.M.K., H.P. and E.H.S. wrote the manuscript. All authors discussed the results and assisted during manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–41, Tables 1–4, Note and refs. 1–3.
Supplementary Data 1
Atomic coordinates of the optimized computational models.
Rights and permissions
About this article
Cite this article
Zhang, B., Wang, L., Cao, Z. et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat Catal 3, 985–992 (2020). https://doi.org/10.1038/s41929-020-00525-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00525-6
This article is cited by
-
High-spin Co3+ in cobalt oxyhydroxide for efficient water oxidation
Nature Communications (2024)
-
Distance effect of single atoms on stability of cobalt oxide catalysts for acidic oxygen evolution
Nature Communications (2024)
-
Highly efficient anion exchange membrane water electrolyzers via chromium-doped amorphous electrocatalysts
Nature Communications (2024)
-
Hierarchical cobalt-molybdenum layered double hydroxide arrays power efficient oxygen evolution reaction
Nano Research (2024)
-
First-Principles Study of Oxygen Evolution Reaction on Ir with Different Coordination Numbers Anchoring at Specific Sites of Co3O4 (111) Surface
Catalysis Letters (2024)