Abstract
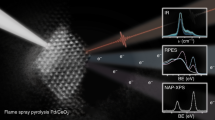
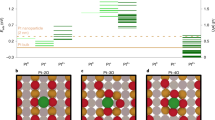
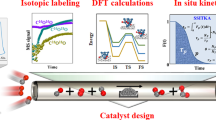
Platinum single sites are highly attractive due to their high atom economy and can be generated on CeO2 by an oxidative high-temperature treatment. However, their location and activity are strongly debated. Furthermore, reaction-driven structural dynamics have not been addressed so far. In this study, we were able to evidence platinum-induced CeO2 surface restructuring, locate platinum single sites on CeO2 and track the variation of the active state under reaction conditions using a complementary approach of density functional theory calculations, in situ infrared spectroscopy, operando high-energy-resolution fluorescence detected X-ray absorption spectroscopy and catalytic CO (as well as C3H6 and CH4) oxidation. We found that the onset of CO oxidation is linked to the migration of platinum single sites from four-fold hollow sites to form small clusters containing a few platinum atoms. This demonstrates that operando studies on single sites are essential to assess their fate and the resulting catalytic properties.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files) or can be obtained from the authors upon reasonable request.
References
Deutschmann, O. & Grunwaldt, J.-D. Exhaust gas after treatment in mobile systems: status, challenges, and perspectives. Chem. Ing. Tech. 85, 595–617 (2013).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Mitchell, S., Vorobyeva, E. & Pérez‐Ramírez, J. The multifaceted reactivity of single‐atom heterogeneous catalysts. Angew. Chem. Int. Ed. 57, 15316–15329 (2018).
Beniya, A. & Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2, 590–602 (2019).
Lin, J. et al. Remarkable performance of Ir1/FeOX single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 135, 15314–15317 (2013).
Neitzel, A. et al. Atomically dispersed Pd, Ni, and Pt species in ceria-based catalysts: principal differences in stability and reactivity. J. Phys. Chem. C 120, 9852–9862 (2016).
Therrien, A. J. et al. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 1, 192–198 (2018).
Chen, Z. et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat. Nanotechnol. 13, 702–707 (2018).
DeRita, L. et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 18, 746–751 (2019).
Tauster, S. J., Fung, S. C. & Garten, R. L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 100, 170–175 (1978).
Haller, G. L. & Resasco, D. E. Metal–support interaction: group VIII metals and reducible oxides. Adv. Catal. 36, 173–235 (1989).
Nagai, Y. et al. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 242, 103–109 (2006).
Farmer, J. A. & Campbell, C. T. Ceria maintains smaller metal catalyst particles by strong metal-support bonding. Science 329, 933–936 (2010).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Daelman, N., Capdevila-Cortada, M. & López, N. Dynamic charge and oxidation state of Pt/CeO2 single-atom catalysts. Nat. Mater. 18, 1215–1221 (2019).
Wang, X., Van Bokhoven, J. A. & Palagin, D. Atomically dispersed platinum on low index and stepped ceria surfaces: phase diagrams and stability analysis. Phys. Chem. Chem. Phys. 22, 28–38 (2019).
Dvořák, F. et al. Creating single-atom Pt-ceria catalysts by surface step decoration. Nat. Commun. 7, 10801 (2016).
Kunwar, D. et al. Stabilizing high metal loadings of thermally stable platinum single atoms on an industrial catalyst support. ACS Catal. 9, 3978–3990 (2019).
Feng, Y. et al. Correlating DFT calculations with CO oxidation reactivity on Ga-doped Pt/CeO2 single-atom catalysts. J. Phys. Chem. C. 122, 22460–22468 (2018).
Ke, J. et al. Strong local coordination structure effects on subnanometer PtOX clusters over CeO2 nanowires probed by low-temperature CO oxidation. ACS Catal. 5, 5164–5173 (2015).
Sayle, T. X. T., Parker, S. C. & Catlow, C. R. A. Surface segregation of metal ions in cerium dioxide. J. Phys. Chem. 98, 13625–13630 (1994).
Bruix, A. et al. Maximum noble-metal efficiency in catalytic materials: atomically dispersed surface platinum. Angew. Chem. Int. Ed. 53, 10525–10530 (2014).
Kottwitz, M. et al. Local structure and electronic state of atomically dispersed Pt supported on nanosized CeO2. ACS Catal. 9, 8738–8748 (2019).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Tang, Y., Wang, Y.-G. & Li, J. Theoretical investigations of Pt1@CeO2 single-atom catalyst for CO oxidation. J. Phys. Chem. C. 121, 11281–11289 (2017).
Bera, P. et al. Promoting effect of CeO2 in combustion synthesized Pt/CeO2 catalyst for CO oxidation. J. Phys. Chem. B 107, 6122–6130 (2003).
Singh, P. & Hegde, M. S. Sonochemical synthesis of thermally stable hierarchical Ce1-xMxO2-δ (M = Pt or Pd, 0 ≤ x ≤ 0.10) nanocrystallites: redox properties and methanol electro-oxidation activity. Cryst. Growth Des. 10, 2995–3004 (2010).
Hegde, M. S. & Bera, P. Noble metal ion substituted CeO2 catalysts: electronic interaction between noble metal ions and CeO2 lattice. Catal. Today 253, 40–50 (2015).
Lee, J., Ryou, Y., Chan, X., Kim, T. J. & Kim, D. H. How Pt Interacts with CeO2 under the reducing and oxidizing environments at elevated temperature: the origin of improved thermal stability of Pt/CeO2 compared to CeO2. J. Phys. Chem. C 120, 25870–25879 (2016).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 341, 771–773 (2013).
Bera, P. et al. Ionic dispersion of Pt over CeO2 by the combustion method: structural investigation by XRD, TEM, XPS, and EXAFS. Chem. Mater. 15, 2049–2060 (2003).
Gatla, S. et al. Facile synthesis of high-surface area platinum-doped ceria for low temperature CO oxidation. Catal. Today 333, 105–112 (2019).
Pereira-Hernández, X. I. et al. Tuning Pt-CeO2 interactions by high-temperature vapor-phase synthesis for improved reducibility of lattice oxygen. Nat. Commun. 10, 1358 (2019).
Wang, H. et al. Surpassing the single-atom catalytic activity limit through paired Pt-O-Pt ensemble built from isolated Pt1 atoms. Nat. Commun. 10, 3808 (2019).
Gänzler, A. M. et al. Tuning the structure of platinum particles on ceria in situ for enhancing the catalytic performance of exhaust gas catalysts. Angew. Chem. Int. Ed. 56, 13078–13082 (2017).
Gorczyca, A. et al. Monitoring morphology and hydrogen coverage of nanometric Pt/γ-Al2O3 particles by in situ HERFD-XANES and quantum simulations. Angew. Chem. Int. Ed. 53, 12426–12429 (2014).
Gänzler, A. M. et al. Operando spatially and time-resolved X-ray absorption spectroscopy and infrared thermography during oscillatory CO oxidation. J. Catal. 328, 216–224 (2015).
Dessal, C. et al. Atmosphere-dependent stability and mobility of catalytic Pt single atoms and clusters on γ-Al2O3. Nanoscale 11, 6897–6904 (2019).
Ye, X. et al. Insight of the stability and activity of platinum single atoms on ceria. Nano Res. 12, 1401–1409 (2019).
Brogan, M. S., Dines, T. J. & Cairns, J. A. Raman spectroscopic study of the Pt–CeO2 interaction in the Pt/Al2O3–CeO2 catalyst. J. Chem. Soc. Faraday Trans. 90, 1461–1466 (1994).
Branda, M. M., Ferullo, R. M., Causà, M. & Illas, F. Relative stabilities of low index and stepped CeO2 surfaces from hybrid and GGA + U implementations of density functional theory. J. Phys. Chem. C. 115, 3716–3721 (2011).
Ding, K. et al. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Chen, A. et al. Structure of the catalytically active copper–ceria interfacial perimeter. Nat. Catal. 2, 334–341 (2019).
Wang, Y. & Wöll, C. IR spectroscopic investigations of chemical and photochemical reactions on metal oxides: bridging the materials gap. Chem. Soc. Rev. 46, 1875–1932 (2017).
Gänzler, A. M. et al. Tuning the Pt/CeO2 interface by in situ variation of the Pt particle size. ACS Catal. 8, 4800–4811 (2018).
Safonova, O. V. et al. Identification of CO adsorption sites in supported Pt catalysts using high-energy-resolution fluorescence detection X-ray spectroscopy. J. Phys. Chem. B 110, 16162–16164 (2006).
Singh, J. & van Bokhoven, J. A. Structure of alumina supported platinum catalysts of different particle size during CO oxidation using in situ IR and HERFD XAS. Catal. Today 155, 199–205 (2010).
Lu, Y. et al. Identification of the active complex for CO oxidation over single-atom Ir-on-MgAl2O4 catalysts. Nat. Catal. 2, 149–156 (2019).
Lang, R. et al. Non defect-stabilized thermally stable single-atom catalyst. Nat. Commun. 10, 234 (2019).
Lott, P., Dolcet, P., Casapu, M., Grunwaldt, J.-D. & Deutschmann, O. The effect of pre-reduction on the performance of Pd/Al2O3 and Pd/CeO2 catalysts during methane oxidation. Ind. Eng. Chem. Res. 58, 12561–12570 (2019).
Cullis, C. F. & Willatt, B. M. Oxidation of methane over supported precious metal catalysts. J. Catal. 83, 267–285 (1983).
Casapu, M. et al. Origin of the normal and inverse hysteresis behavior during CO oxidation over Pt/Al2O3. ACS Catal. 7, 343–355 (2017).
Abedi, A., Hayes, R., Votsmeier, M. & Epling, W. S. Inverse hysteresis phenomena during CO and C3H6 oxidation over a Pt/Al2O3 catalyst. Catal. Lett. 142, 930–935 (2012).
de Juan, A., Jaumot, J. & Tauler, R. Multivariate curve resolution (MCR). Solving the mixture analysis problem. Anal. Methods 6, 4964–4976 (2014).
Jaumot, J., de Juan, A. & Tauler, R. MCR-ALS GUI 2.0: new features and applications. Chemometr. Intell. Lab. Syst. 140, 1–12 (2015).
Voronov, A. et al. Multivariate curve resolution applied to in situ X-ray absorption spectroscopy data: an efficient tool for data processing and analysis. Anal. Chim. Acta 840, 20–27 (2014).
Parkinson, G. S. et al. Carbon monoxide-induced adatom sintering in a Pd–Fe3O4 model catalyst. Nat. Mater. 12, 724–728 (2013).
Kleist, W. & Grunwaldt, J.-D. High Output Catalyst Development in Heterogeneous Gas Phase Catalysis. In Modern Applications of High Throughput R&D in Heterogeneous Catalysis (eds Hagemeyer, A. & Volpe, A. F.) 357–371 (BENTHAM SCIENCE PUBLISHERS, 2014).
Brunauer, S., Emmett, P. H. & Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Grunwaldt, J.-D., Caravati, M., Hannemann, S. & Baiker, A. X-ray absorption spectroscopy under reaction conditions: suitability of different reaction cells for combined catalyst characterization and time-resolved studies. Phys. Chem. Chem. Phys. 6, 3037–3047 (2004).
Bunău, O. & Joly, Y. Self-consistent aspects of X-ray absorption calculations. J. Phys. Condens. Matter 21, 345501 (2009).
Yan, H. et al. Atomic engineering of high-density isolated Co atoms on graphene with proximal-atom controlled reaction selectivity. Nat. Commun. 9, 3197 (2018).
Timoshenko, J., Lu, D., Lin, Y. & Frenkel, A. I. Supervised machine-learning-based determination of three-dimensional structure of metallic nanoparticles. J. Phys. Chem. Lett. 8, 5091–5098 (2017).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Bahn, S. R. & Jacobsen, K. W. An object-oriented scripting interface to a legacy electronic structure code. Comput. Sci. Eng. 4, 56–66 (2002).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Wellendorff, J. et al. Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys. Rev. B 85, 235149 (2012).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Fairley, N. CasaXPS Manual 2.3.16: Introduction to XPS and AES (Casa Software, 2011).
Acknowledgements
F.M. (ITCP, KIT) thanks the “Fonds der Chemischen Industrie” (FCI) for financial support. J.J. and F.S. acknowledge support by the state of Baden-Württemberg through bwHPC (bwunicluster and JUSTUS, RV bw16G001 and bwl17D011). J.W. is grateful for a PhD fellowship, donated by the China Scholarship Council (CSC). The authors further thank the German Federal Ministry for Economic Affairs and Energy (BMWi: 19U15014B) and the French National Research Agency (ANR‐14‐CE22‐0011‐02) for financial support of the ORCA project within the DEUFRAKO program, the DFG for financial support (INST 121384/16-1, INST 121384/73-1, INST 121384/73-1) and DESY and ESRF for beamtime at the P65 and BM16 beamlines, respectively. We thank D. Zengel, P. Lott, G. Cavusoglu and D. Doronkin (ITCP/IKFT, KIT) for assistance during operando XAS and HERFD-XANES experiments. Furthermore, we acknowledge M. Stehle, A. Deutsch and J. Pesek (ITCP, KIT) for technical support with respect to catalyst preparation, characterization and testing as well as T. Bergfeldt (IAM-AWP, KIT) and H. Störmer (LEM, KIT) for ICP-OES analysis and HAADF-STEM, respectively. Finally, M. Rovezzi, A. Aguilar (BM16, ESRF), E. Welter, R. Nemausat and M. Herrmann (P65, DESY) are thanked for support during beamtime at the corresponding beamlines. We also thank A. Zitolo (Samba, SOLEIL) for fruitful discussion on XANES data calculations.
Author information
Authors and Affiliations
Contributions
F.M. performed the catalyst preparation, X-ray-based characterization, FEFF calculations, catalytic tests and wrote the paper. J.J. designed and performed the DFT calculations together with F.S. and wrote the corresponding text in the paper. J.W., Y.W. and C.W. conducted the UHV-FTIRS and XPS characterization and interpretation. A.G. was involved in the DRIFTS and HERFD-XAS experiments as well as catalyst preparation. P.D. performed further catalytic tests, the FDMNES calculations and helped in the FEFF calculations as well as the analysis of the EXAFS and HERFD-XANES data. M.C. and J.-D.G. designed the study and co-wrote the paper. All the authors contributed to the interpretation of the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion and methods (with interdispersed Supplementary Figs. 1–30 and Tables 1–13) and references.
Supplementary Data
Contains the CONTCAR files of the VASP calculations for all structures mentioned in the manuscript and Supplementary Information.
Rights and permissions
About this article
Cite this article
Maurer, F., Jelic, J., Wang, J. et al. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nat Catal 3, 824–833 (2020). https://doi.org/10.1038/s41929-020-00508-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00508-7
This article is cited by
-
Interrogating site dependent kinetics over SiO2-supported Pt nanoparticles
Nature Communications (2024)
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)
-
Memory-dictated dynamics of single-atom Pt on CeO2 for CO oxidation
Nature Communications (2023)
-
Single-atomic platinum on fullerene C60 surfaces for accelerated alkaline hydrogen evolution
Nature Communications (2023)
-
Activating dynamic atomic-configuration for single-site electrocatalyst in electrochemical CO2 reduction
Nature Communications (2023)