Abstract
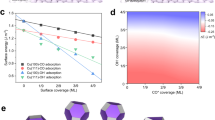
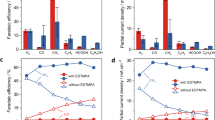
Electrochemical CO2 reduction to value-added chemical feedstocks is of considerable interest for renewable energy storage and renewable source generation while mitigating CO2 emissions from human activity. Copper represents an effective catalyst in reducing CO2 to hydrocarbons or oxygenates, but it is often plagued by a low product selectivity and limited long-term stability. Here we report that copper nanowires with rich surface steps exhibit a remarkably high Faradaic efficiency for C2H4 that can be maintained for over 200 hours. Computational studies reveal that these steps are thermodynamically favoured compared with Cu(100) surface under the operating conditions and the stepped surface favours C2 products by suppressing the C1 pathway and hydrogen production.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Schreier, M. et al. Solar conversion of CO2 to CO using Earth-abundant electrocatalysts prepared by atomic layer modification of CuO. Nat. Energy 2, 17087 (2017).
Hori, Y., Wakebe, H., Tsukamoto, T. & Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 39, 1833–1839 (1994).
Hori, Y., Kikuchi, K. & Suzuki, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogen carbonate solution. Chem. Lett. 14, 1695–1698 (1985).
Qiao, J., Liu, Y., Hong, F. & Zhang, J. A review of catalysts for the electro-reduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 43, 631–675 (2014).
Gawande, M. B. et al. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev. 116, 3722–3811 (2016).
Kim, D., Resasco, J., Yu, Y., Asiri, A. M. & Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 5, 4948 (2014).
Lu, Q. et al. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 5, 3242 (2014).
Mistry, H., Varela, A. S., Kühl, S., Strasser, P. & Cuenya, B. R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1, 16009 (2016).
Angamuthu, R., Byers, P., Lutz, M., Spek, A. L. & Bouwman, E. Electrocatalytic CO2 conversion to oxalate by a copper complex. Science 327, 313–315 (2010).
Li, Y. et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 17, 1312–1317 (2017).
Cheng, T., Xiao, H. & Goddard, W. A. III Reaction mechanisms for the electrochemical reduction of CO2 to CO and formate on the Cu(100) surface at 298 K from quantum mechanics free energy calculations with explicit water. J. Am. Chem. Soc. 138, 13802–13805 (2016).
Raciti, D., Mao, M., Park, J. H. & Wang, C. Local pH effect in the CO2 reduction reaction on high-surface-area copper electrocatalysts. J. Electrochem. Soc. 165, F799 (2018).
Li, C. W. & Kanan, M. W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 134, 7231–7234 (2012).
Mistry, H. et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 7, 12123 (2016).
Choi, C. et al. A highly active star decahedron Cu nanocatalyst for hydrocarbon production at low overpotentials. Adv. Mater. 31, 1805405 (2019).
Feng, X., Jiang, K., Fan, S. & Kanan, M. W. Grain-boundary-dependent CO2 electroreduction activity. J. Am. Chem. Soc. 137, 4606–4609 (2015).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Mariano, R. G., McKelvey, K., White, H. S. & Kanan, M. W. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science 358, 1187–1192 (2017).
Favaro, M. et al. Subsurface oxide plays a critical role in CO2 activation by Cu(111) surfaces to form chemisorbed CO2, the first step in reduction of CO2. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1701405114 (2017).
Lum, Y. & Ager, J. W. Stability of residual oxides in oxide‐derived copper catalysts for electrochemical CO2 reduction investigated with 18O labeling. Angew. Chem. Int. Ed. Engl. 57, 551–554 (2018).
Ethylene—Global Market Trajectory and Analytics https://www.researchandmarkets.com/reports/354876/ethylene_global_market_trajectory_and_analytics (Research and Markets, 2020).
Cheng, T., Xiao, H. & Goddard, W. A. Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298 K. Proc. Natl Acad. Sci. USA 114, 1795–1800 (2017).
Cheng, T., Xiao, H. & Goddard, W. A. Nature of the active sites for CO reduction on copper nanoparticles; suggestions for optimizing performance. J. Am. Chem. Soc. 139, 11642–11645 (2017).
Hori, Y., Takahashi, I., Koga, O. & Hoshi, N. Selective formation of C2 compounds from electrochemical reduction of CO2 at a series of copper single crystal electrodes. J. Phys. Chem. B 106, 15–17 (2002).
Jin, M. et al. Shape‐controlled synthesis of copper nanocrystals in an aqueous solution with glucose as a reducing agent and hexadecylamine as a capping agent. Angew. Chem. Int. Ed. 50, 10560–10564 (2011).
Yang, H. J., He, S. Y. & Tuan, H. Y. Self-seeded growth of five-fold twinned copper nanowires: mechanistic study, characterization, and SERS applications. Langmuir 30, 602–610 (2014).
Mandal, L. et al. Investigating the role of copper oxide in electrochemical CO2 reduction in real time. ACS Appl. Mater. Inter. 10, 8574–8584 (2018).
Baturina, O. A. et al. CO2 electroreduction to hydrocarbons on carbon-supported Cu nanoparticles. ACS Catal. 4, 3682–3695 (2014).
Droog, J. M. & Schlenter, B. Oxygen electrosorption on copper single crystal electrodes in sodium hydroxide solution. J. Electroanal. Chem. 112, 387–390 (1980).
De Chialvo, M. G., Zerbino, J. O., Marchiano, S. L. & Arvia, A. J. Correlation of electrochemical and ellipsometric data in relation to the kinetics and mechanism of Cu2O electroformation in alkaline solutions. J. Appl. Electrochem. 16, 517–526 (1986).
Raciti, D. et al. Low-overpotential electroreduction of carbon monoxide using copper nanowires. ACS Catal. 7, 4467–4472 (2017).
Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 1, 423–430 (2019).
De Chialvo, M. G., Marchiano, S. L. & Arvia, A. J. The mechanism of oxidation of copper in alkaline solutions. J. Appl. Electrochem. 14, 165–175 (1984).
Zhang, S., Kang, P. & Meyer, T. J. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Am. Chem. Soc. 136, 1734–1737 (2014).
Tian, F. H. & Wang, Z. X. Adsorption of an O atom on the Cu(311) step defective surface. J. Phys. Chem. B 108, 1392–1395 (2004).
Hori, Y., Wakebe, H., Tsukamoto, T. & Koga, O. Adsorption of CO accompanied with simultaneous charge transfer on copper single crystal electrodes related with electrochemical reduction of CO2 to hydrocarbons. Surf. Sci. 335, 258–263 (1995).
Baricuatro, J. H., Kim, Y. G., Korzeniewski, C. L. & Soriaga, M. P. Seriatim ECSTM–ECPMIRS of the adsorption of carbon monoxide on Cu(100) in alkaline solution at CO2-reduction potentials. Electrochem. Commun. 91, 1–4 (2018).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Montoya, J. H., Shi, C., Chan, K. & Nørskov, J. K. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 6, 2032–2037 (2015).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 13, 4998 (2017).
Yamamoto, S. et al. In situ X-ray photoelectron spectroscopy studies of water on metals and oxides at ambient conditions. J. Phys. Condens. Matter 20, 184025 (2008).
Xiao, H., Cheng, T. & Goddard, W. A. III Atomistic mechanisms underlying selectivities in C1 and C2 products from electrochemical reduction of CO on Cu(111). J. Am. Chem. Soc. 139, 130–136 (2016).
DeWulf, D. W., Jin, T. & Bard, A. J. Electrochemical and surface studies of carbon dioxide reduction to methane and ethylene at copper electrodes in aqueous solutions. J. Electrochem. Soc. 136, 1686–1691 (1989).
Engelbrecht, A. et al. On the electrochemical CO2 reduction at copper sheet electrodes with enhanced long-term stability by pulsed electrolysis. J. Electrochem. Soc. 165, J3059–J3068 (2018).
Zhu, W. et al. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 135, 16833–16836 (2013).
Kresse, G., Furthmüller, J. & Hafner, J. Theory of the crystal structures of selenium and tellurium: the effect of generalized-gradient corrections to the local-density approximation. Phys. Rev. B 50, 13181 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle point. J. Chem. Phys. 113, 9978 (2000).
Gao, D. et al. Plasma-activated copper nanocube catalysts for efficient carbon dioxide electroreduction to hydrocarbons and alcohols. ACS Nano 11, 4825–4831 (2017).
Kim, D., Kley, C. S., Li, Y. & Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl Acad. Sci. USA 114, 10560–10565 (2017).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Kim, J. et al. Branched copper oxide nanoparticles induce highly selective ethylene production by electrochemical carbon dioxide reduction. J. Am. Chem. Soc. 141, 6986–6994 (2019).
Jung, H. et al. Electrochemical fragmentation of Cu2O nanoparticles enhancing selective C–C coupling from CO2 reduction reaction. J. Am. Chem. Soc. 141, 4624–4633 (2019).
Acknowledgements
The TEM work was conducted using the facilities in the Electron Imaging Center at the California NanoSystems Institute at the University of California Los Angles and the Irvine Materials Research Institute at the University of California Irvine. C.C., J.C., X.D. and Y.H. acknowledge support from the Office of Naval Research (ONR) under grant no. N000141712608. S.K., T.C. and W.A.G. were supported by the Joint Center for Artificial Photosynthesis, a DOE Energy Innovation Hub, supported through the Office of Science of the US Department of Energy under Award no. DE-SC0004993. C.L., S.K. and H.M.L. used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant no. ACI-1548562. C.L. and H.M.L. were also supported by a National Research Foundation (NRF) of Korea grant funded by the Korean Government (no. NRF-2017R1E1A1A03071049). The work done at the University of California Irvine was supported by the Irvine Materials Research Institute and ExxonMobil.
Author information
Authors and Affiliations
Contributions
C.C. designed and conducted most of the experiments, analysed all the data and prepared the manuscript. S.K., T.C. and W.A.G. performed the density theoretical calculations and prepared the manuscript. M.X., P.T. and X.P. took SEI and bright-field scanning transmission electron microscopy images. J.C., C.L., H.M.L and X.D. assisted in the experiments and the preparation of the manuscript. Y.H. initiated the study, oversaw the project and wrote the manuscript. All the authors discussed the results and contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15, Tables 1–6 and references.
Supplementary Data 1
Atomic structures of the initial state, transition state and final state
Rights and permissions
About this article
Cite this article
Choi, C., Kwon, S., Cheng, T. et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat Catal 3, 804–812 (2020). https://doi.org/10.1038/s41929-020-00504-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00504-x
This article is cited by
-
Self-supporting BiCu/carbon hybrid nanofiber membrane promotes efficient CO2 electroreduction to formate
Science China Materials (2024)
-
Combined effects of sea urchin-like structure and mixed Cu+/Cu0 states on promoting C2 formation in electrocatalytic CO2 reduction
Frontiers of Chemical Science and Engineering (2024)
-
Efficient multicarbon formation in acidic CO2 reduction via tandem electrocatalysis
Nature Nanotechnology (2024)
-
Surface hydroxide promotes CO2 electrolysis to ethylene in acidic conditions
Nature Communications (2023)
-
Oxidation of metallic Cu by supercritical CO2 and control synthesis of amorphous nano-metal catalysts for CO2 electroreduction
Nature Communications (2023)