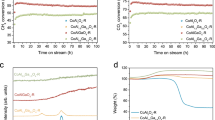
Abstract
Dry reforming of methane usually affords low-quality syngas with equimolar amounts of CO and H2. Here we report the high conversion of CH4 and CO2 to syngas and solid carbon through simultaneous pyrolysis and dry reforming of methane in a bubble column reactor using a molten metal alloy catalyst (65:35 mol% Ni:In). The H2 to CO ratio can be increased above 1:1 using feed ratios of CH4:CO2 greater than 1:1 to produce stoichiometric solid carbon as a co-product that is separable from the molten metal. A coupled reduction–oxidation cycle is carried out in which CO2 is reduced by a liquid metal species (for example, In) and methane is partially oxidized to syngas by the metal oxide intermediate (for example, In2O3), regenerating the native metal. Moreover, the H2:CO product ratio can be easily controlled by adjusting the CH4:CO2 feed ratio, temperature, and residence time in the reactor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author on reasonable request.
References
Pakhare, D. & Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 43, 7813–7837 (2014).
Agrafiotis, C., von Storch, H., Roeb, M. & Sattler, C. Solar thermal reforming of methane feedstocks for hydrogen and syngas production—a review. Renew. Sustain. Energy Rev. 29, 656–682 (2014).
Lau, C. S., Tsolakis, A. & Wyszynski, M. L. Biogas upgrade to syn-gas (H2–CO) via dry and oxidative reforming. Int. J. Hydrog. Energy 36, 397–404 (2011).
Henrici-Olivé, G. & Olivé, S. The Fischer–Tropsch synthesis: molecular weight distribution of primary products and reaction mechanism. Angew. Chem. Int. Ed. 15, 136–141 (1976).
Smith, A. R. & Klosek, J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process. Technol. 70, 115–134 (2001).
Rostrup-Nielsen, J. R., Sehested, J. & Nørskov, J. K. Hydrogen and synthesis gas by steam- and CO2 reforming. Adv. Catal. 47, 65–139 (2002).
Bradford, M. C. J. & Vannice, M. A. CO2 reforming of CH4. Catal. Rev. Sci. Eng. 41, 1–42 (1999).
Horn, R. & Schlogl, R. Methane activation by heterogeneous catalysis. Catal. Lett. 145, 23–29 (2015).
Nikoo, M. K. & Amin, N. A. S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 92, 678–691 (2011).
Rostrup-Nielsen, J. R. Industrial relevance of coking. Catal. Today 37, 225–232 (1997).
Li, Y., Wang, Y., Zhang, X. & Mi, Z. Thermodynamic analysis of autothermal steam and CO2 reforming of methane. Int. J. Hydrog. Energy 33, 2507–2514 (2008).
Rostrup-Nielsen, J. R. Production of synthesis gas. Catal. Today 18, 305–324 (1993).
Song, C. S. & Wei, P. Tri-reforming of methane: a novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios. Catal. Today 98, 463–484 (2004).
Aasberg-Petersen, K. et al. Natural gas to synthesis gas—catalysts and catalytic processes. J. Nat. Gas. Sci. Eng. 3, 423–459 (2011).
Fan, M. S., Abdullah, A. Z. & Bhatia, S. Catalytic technology for carbon dioxide reforming of methane to synthesis gas. ChemCatChem 1, 192–208 (2009).
Li, Z., Mo, L., Kathiraser, Y. & Kawi, S. Yolk–satellite–shell structured Ni–yolk@Ni@SiO2 nanocomposite: superb catalyst toward methane CO2 reforming reaction. ACS Catal. 4, 1526–1536 (2014).
Kim, S. M. et al. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 139, 1937–1949 (2017).
Li, X. Y. et al. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B 202, 683–694 (2017).
Das, S. et al. Silica–ceria sandwiched Ni core-shell catalyst for low temperature dry reforming of biogas: coke resistance and mechanistic insights. Appl. Catal. B 230, 220–236 (2018).
Tu, X. & Whitehead, J. C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: understanding the synergistic effect at low temperature. Appl. Catal. B 125, 439–448 (2012).
Tu, X. & Whitehead, J. C. Plasma dry reforming of methane in an atmospheric pressure AC gliding arc discharge: co-generation of syngas and carbon nanomaterials. Int. J. Hydrog. Energy 39, 9658–9669 (2014).
Yagi, F. et al. in Studies in Surface Science and Catalysis (eds Noronha, F. B. et al.) Vol. 167, 385–390 (Elsevier, 2007).
Zhang, P., Tong, J. & Huang, K. Combining electrochemical CO2 capture with catalytic dry methane reforming in a single reactor for low-cost syngas production. ACS Sustain. Chem. Eng. 4, 7056–7065 (2016).
Lu, J. et al. Highly efficient electrochemical reforming of CH4/CO2 in a solid oxide electrolyser. Sci. Adv. 4, eaar5100 (2018).
Steinberg, M. Fossil fuel decarbonization technology for mitigating global warming. Int. J. Hydrog. Energy 24, 771–777 (1999).
Geißler, T. et al. Experimental investigation and thermo-chemical modeling of methane pyrolysis in a liquid metal bubble column reactor with a packed bed. Int. J. Hydrog. Energy 40, 14134–14146 (2015).
Plevan, M. et al. Thermal cracking of methane in a liquid metal bubble column reactor: experiments and kinetic analysis. Int. J. Hydrog. Energy 40, 8020–8033 (2015).
Upham, D. C. et al. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 358, 917–921 (2017).
Wang, K., Li, W. S. & Zhou, X. P. Hydrogen generation by direct decomposition of hydrocarbons over molten magnesium. J. Mol. Catal. A 283, 153–157 (2008).
Palmer, C. et al. Methane pyrolysis with a molten Cu–Bi alloy catalyst. ACS Catal. 9, 8337–8345 (2019).
Kodama, T., Koyanagi, T., Shimizu, T. & Kitayama, Y. CO2 reforming of methane in a molten carbonate salt bath for use in solar thermochemical processes. Energy Fuels 15, 60–65 (2001).
Gokon, N., Oku, Y., Kaneko, H. & Tamaura, Y. Methane reforming with CO2 in molten salt using FeO catalyst. Sol. Energy 72, 243–250 (2002).
Al-Ali, K., Kodama, S. & Sekiguchi, H. Modeling and simulation of methane dry reforming in direct-contact bubble reactor. Sol. Energy 102, 45–55 (2014).
Alberto, G. et al. Solar steam reforming of natural gas for hydrogen production using molten salt heat carriers. AIChE J. 54, 1932–1944 (2008).
Kodama, T., Gokon, N., Inuta, S.-i, Yamashita, S. & Seo, T. Molten-salt tubular absorber/reformer (MoSTAR) project: the thermal storage media of Na2CO3–MgO composite materials. J. Sol. Energy Eng. 131, 041013–041013-8 (2009).
Kodama, T., Isobe, Y., Kondoh, Y., Yamaguchi, S. & Shimizu, K. I. Ni/ceramic/molten-salt composite catalyst with high-temperature thermal storage for use in solar reforming processes. Energy 29, 895–903 (2004).
Bale, C. W. et al. FactSage thermochemical software and databases. Calphad 54, 35–53 (2010-2016).
Oyama, S. T., Hacarlioglu, P., Gu, Y. & Lee, D. Dry reforming of methane has no future for hydrogen production: comparison with steam reforming at high pressure in standard and membrane reactors. Int. J. Hydrog. Energy 37, 10444–10450 (2012).
Otsuka, K., Yasui, T. & Morikawa, A. Reproducible hydrogen production from water by indium oxide. J. Catal. 72, 392–393 (1981).
Otsuka, K., Takizawa, Y., Shibuya, S.-i & Morikawa, A. Hydrogen production from water by In2O3 and K2CO3 using graphite, active carbon and biomass as reductants. Chem. Lett. 10, 347–350 (1981).
Otsuka, K., Takizawa, Y. & Morikawa, A. Hydrogen production from water on carbon-reduced indium oxide. Fuel Process. Technol. 6, 215–223 (1982).
Otsuka, K., Yasui, T. & Morikawa, A. The decomposition of water on the CO- or H2-reduced indium oxide. Bull. Chem. Soc. Jpn 55, 1768–1771 (1982).
Otsuka, K., Shibuya, S.-i & Morikawa, A. Effective supported-In2O3 for the production of hydrogen from water by the reduction-oxidation cycle of In2O3. Chem. Lett. 11, 987–990 (1982).
Otsuka, K., Yasui, T. & Morikawa, A. Production of CO from CO2 by reduced indium oxide. J. Chem. Soc. Faraday Trans. 78, 3281–3286 (1982).
Otsuka, K., Shibuya, S.-I. & Morikawa, A. Role of carriers in the production of hydrogen from water by reduction-oxidation cycle of In2O3. J. Catal. 84, 308–316 (1983).
Jerng, S. K. et al. Nanocrystalline graphite growth on sapphire by carbon molecular beam epitaxy. J. Phys. Chem. C 115, 4491–4494 (2011).
Ferrari, A. C. & Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. Lond. A 362, 2477–2512 (2004).
Pinilla, J. L. et al. Hydrogen production by thermo-catalytic decomposition of methane: regeneration of active carbons using CO2. J. Power Sources 169, 103–109 (2007).
Acknowledgements
Funding to support this work was provided by the Energy & Biosciences Institute through the EBI-Shell programme. Support for S.S. was provided by the Dow Centre for Sustainable Engineering Innovation at the University of Queensland. We made use of Center for Scientific Computing at the California NanoSystems Institute funded in part by NSF CNS-0960316 and Hewlett-Packard. The MRL Shared Experimental Facilities are supported by the MRSEC Program of the NSF under award no. DMR 1720256; a member of the NSF-funded Materials Research Facilities Network (www.mrfn.org). The authors are grateful for the indispensable technical assistance of R. Bock of the UCSB Chemistry Department, who prepared all of the quartz reactor components and their modifications.
Author information
Authors and Affiliations
Contributions
C.P., D.C.U. and E.W.M. conceived the research. C.P. performed the thermodynamic analysis in the main manuscript, experimental work and carbon characterizations, with contributions and feedback from S.S., E.W.M., M.J.G. and H.M. S.S. performed the supplemental thermodynamic analysis. C.P. prepared the data figures. All authors contributed to the written text and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1, Figs. 1–8 and Notes 1–4.
Rights and permissions
About this article
Cite this article
Palmer, C., Upham, D.C., Smart, S. et al. Dry reforming of methane catalysed by molten metal alloys. Nat Catal 3, 83–89 (2020). https://doi.org/10.1038/s41929-019-0416-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0416-2
This article is cited by
-
Binuclear Cu complex catalysis enabling Li–CO2 battery with a high discharge voltage above 3.0 V
Nature Communications (2023)
-
Dry reforming of methane over gallium-based supported catalytically active liquid metal solutions
Communications Chemistry (2023)
-
Current state and future prospects of liquid metal catalysis
Nature Catalysis (2023)
-
Thermal steam methane reforming over bimetal-loaded hemp-derived activated carbon-based catalyst for hydrogen production
Research on Chemical Intermediates (2023)
-
Multiphase flow physics of room temperature liquid metals and its applications
Science China Technological Sciences (2023)